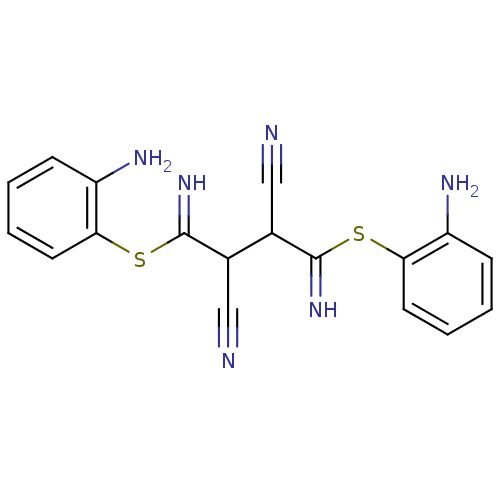
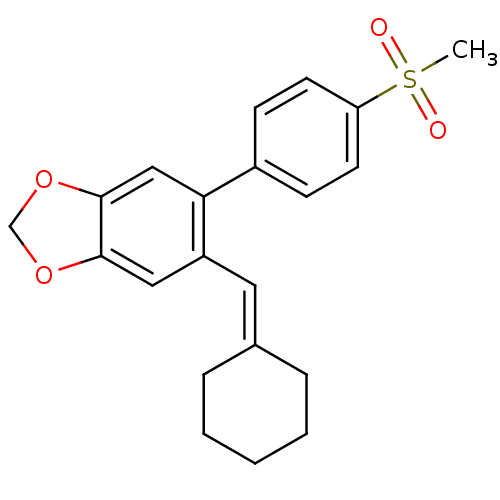
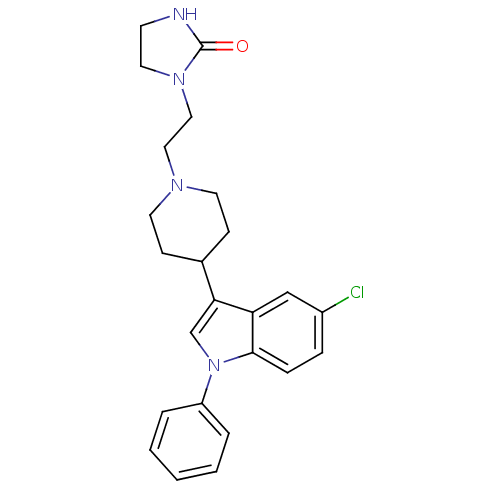
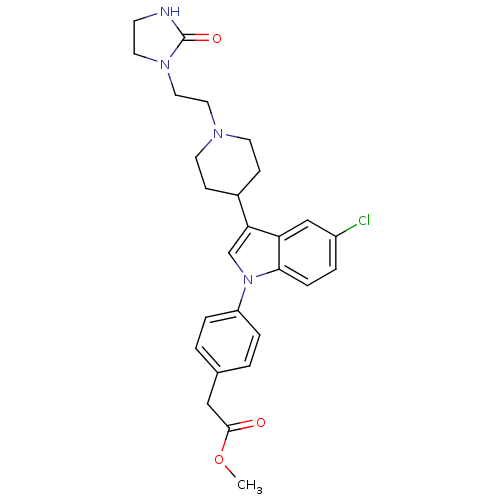
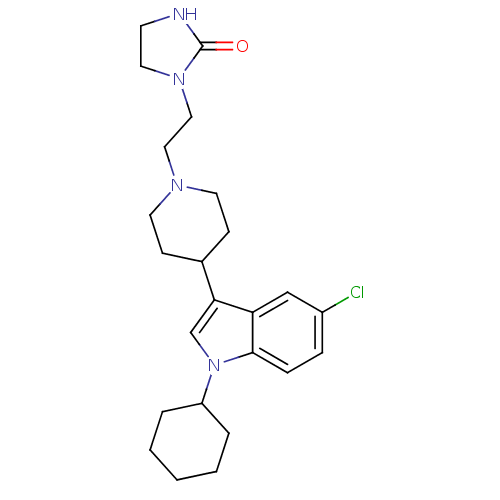
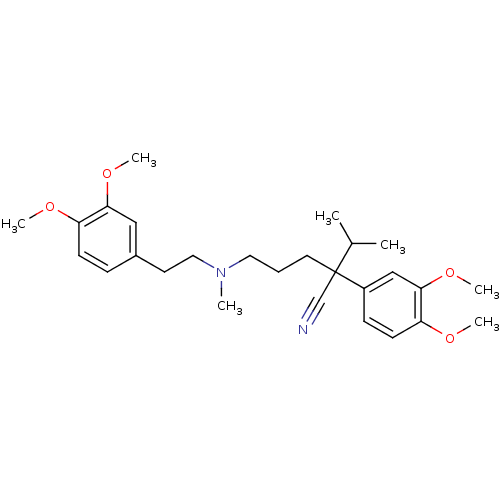
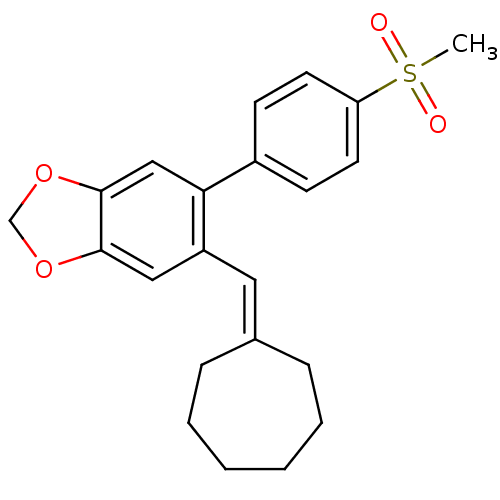
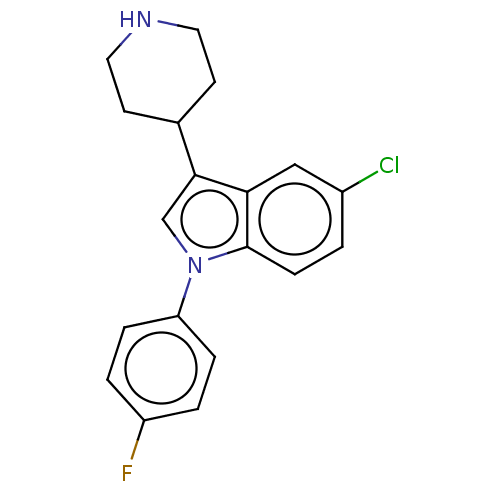
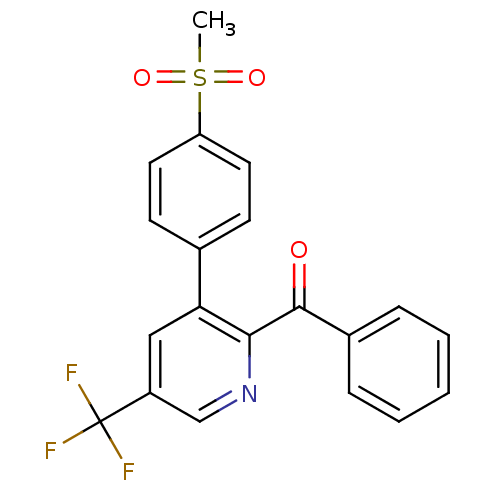
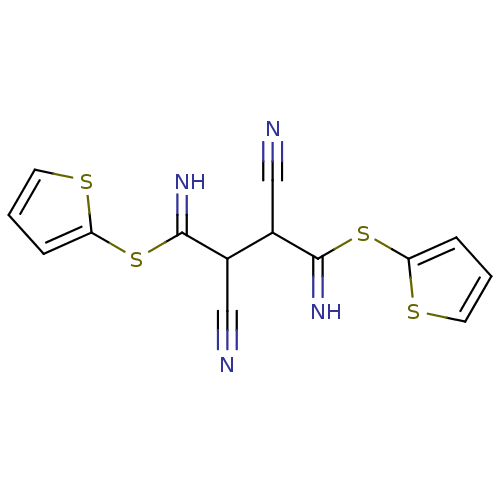
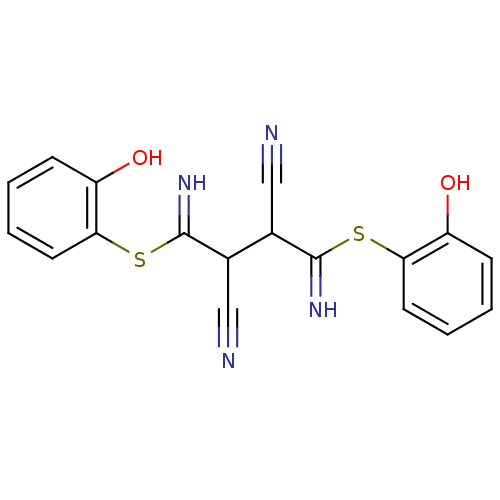
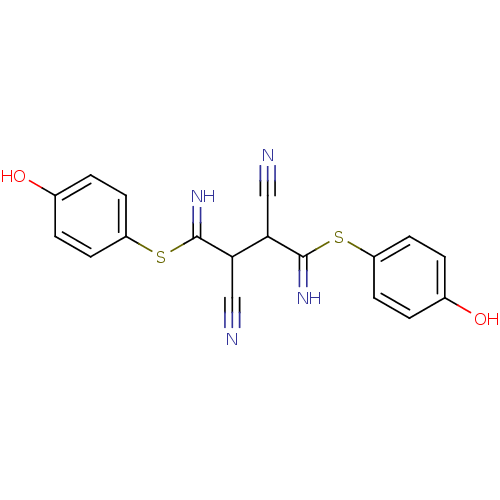
Affinity DataKi: 0.930nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 4.5nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 5.70nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 6.60nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 8.20nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:The compound was evaluated for the binding affinity against bovine pancreatic chymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataKi: 101nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6.20nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 36nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:Compound was tested for the inhibition of recombinant human Prostaglandin G/H synthase 2 (COX-2)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 56nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 2(Homo sapiens (Human))
DuPont Pharmaceuticals Company
Curated by ChEMBL
DuPont Pharmaceuticals Company
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of the dual specificity kinase MEK-2More data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Compound was tested for the inhibition of recombinant human Prostaglandin G/H synthase 2 (COX-2) by enzyme AssayMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Homo sapiens (Human))
DuPont Pharmaceuticals Company
Curated by ChEMBL
DuPont Pharmaceuticals Company
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of the dual specificity kinase MEK-1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 88nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 131nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 137nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 143nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole bloodMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1/2(Homo sapiens (Human))
DuPont Pharmaceuticals Company
Curated by ChEMBL
DuPont Pharmaceuticals Company
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:AP-1 suppression activity using freshly prepared DMSO stockMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 204nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:In vitro inhibitory concentration against Prostaglandin G/H synthase 2 (COX-2) in human whole bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Compound was evaluated for inhibition concentration of prostaglandin G/H synthase 2 in human bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:In vitro inhibition of Prostaglandin G/H synthase 2 in human whole bloodMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Homo sapiens (Human))
DuPont Pharmaceuticals Company
Curated by ChEMBL
DuPont Pharmaceuticals Company
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Non-competitive inhibition of the dual specificity kinase MEKMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Homo sapiens (Human))
DuPont Pharmaceuticals Company
Curated by ChEMBL
DuPont Pharmaceuticals Company
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Non-competitive inhibition of the dual specificity kinase MEKMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1/2(Homo sapiens (Human))
DuPont Pharmaceuticals Company
Curated by ChEMBL
DuPont Pharmaceuticals Company
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:AP-1 suppression activity using one week old DMSO stockMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1/2(Homo sapiens (Human))
DuPont Pharmaceuticals Company
Curated by ChEMBL
DuPont Pharmaceuticals Company
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:AP-1 suppression activity using one month old DMSO stockMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole bloodMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Homo sapiens (Human))
DuPont Pharmaceuticals Company
Curated by ChEMBL
DuPont Pharmaceuticals Company
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Non-competitive inhibition of the dual specificity kinase MEKMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Compound was evaluated for inhibition concentration of prostaglandin G/H synthase 2 in human bloodMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1/2(Homo sapiens (Human))
DuPont Pharmaceuticals Company
Curated by ChEMBL
DuPont Pharmaceuticals Company
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Ability to antagonise AP-1 transcriptional activityMore data for this Ligand-Target Pair


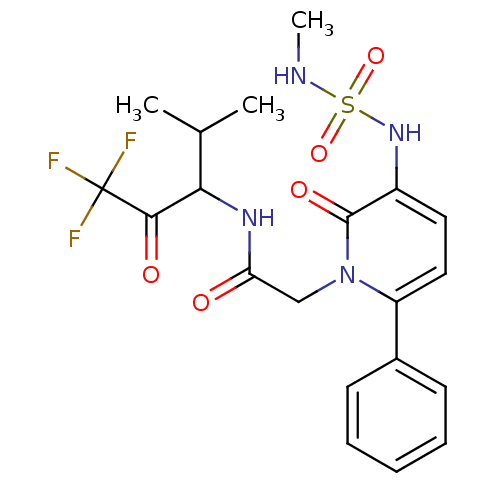
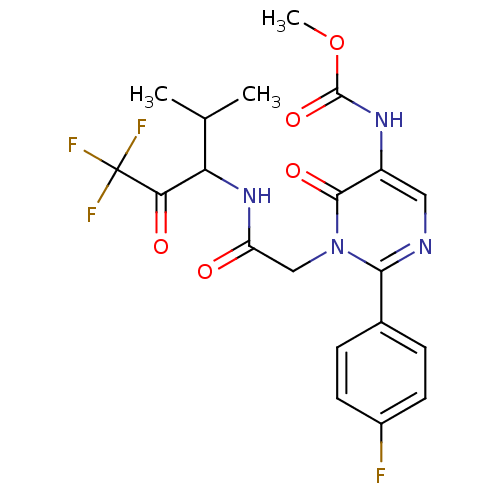
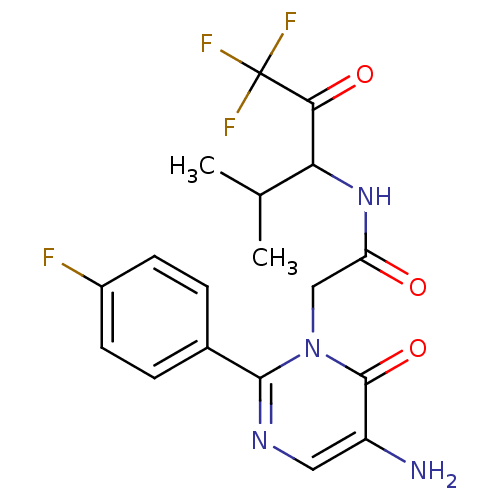
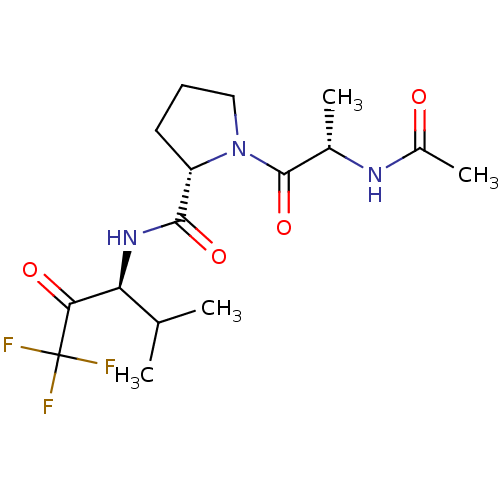
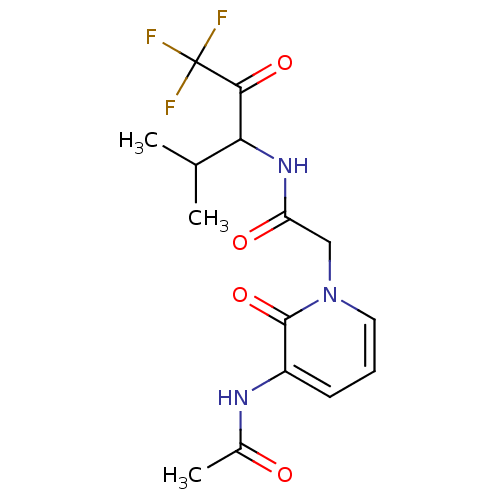
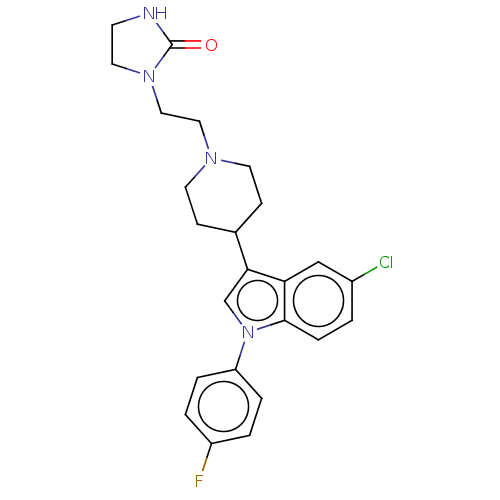
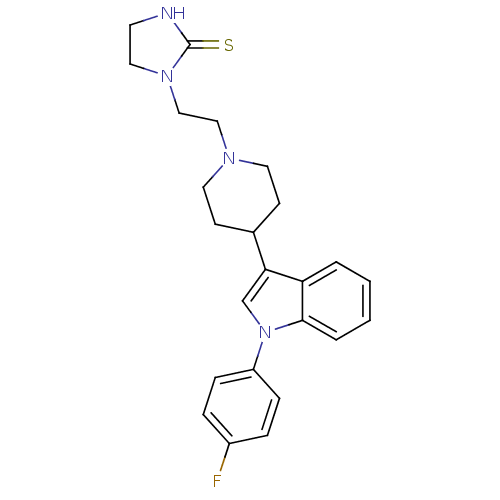
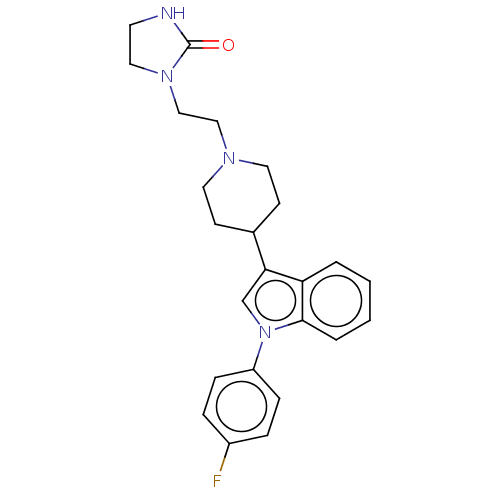





 3D Structure (crystal)
3D Structure (crystal)