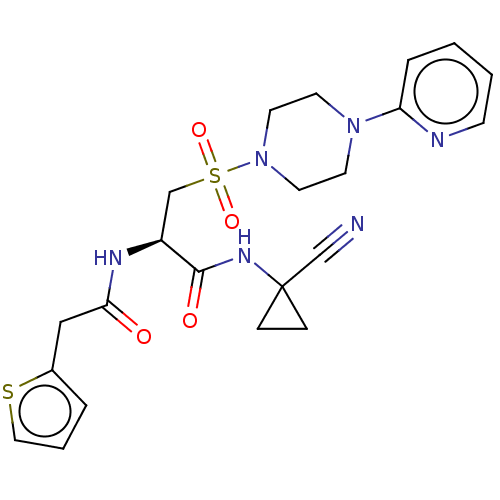
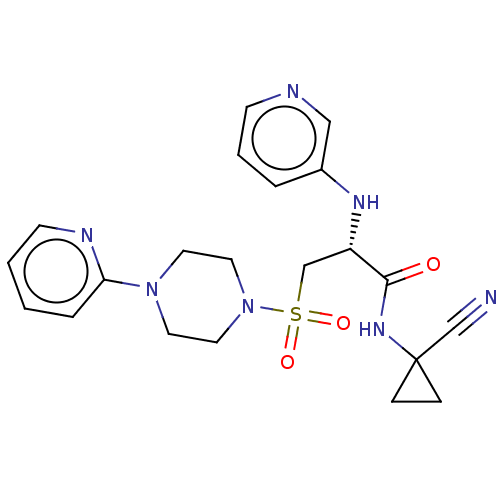
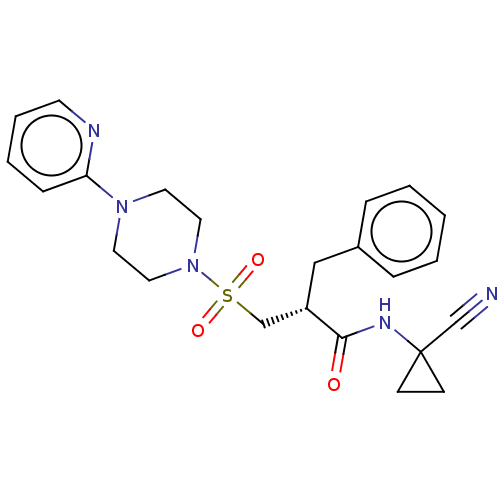
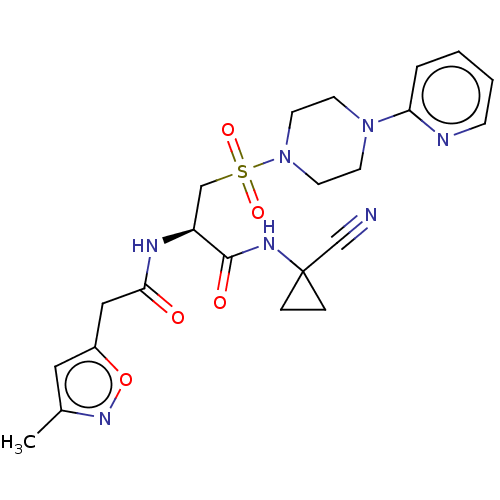
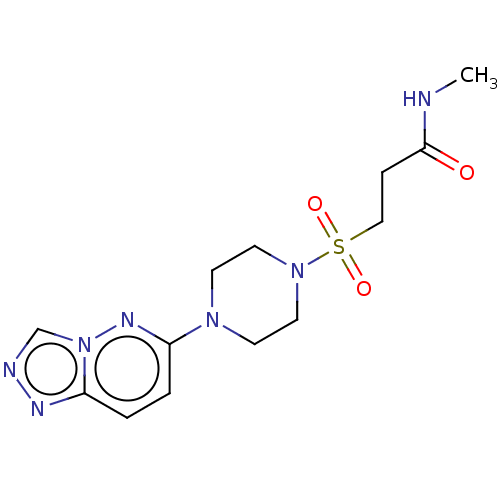
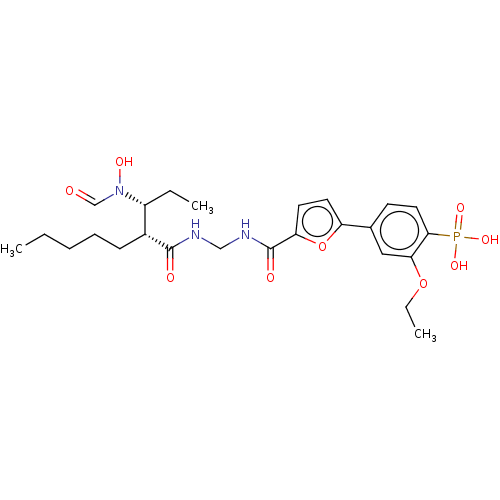
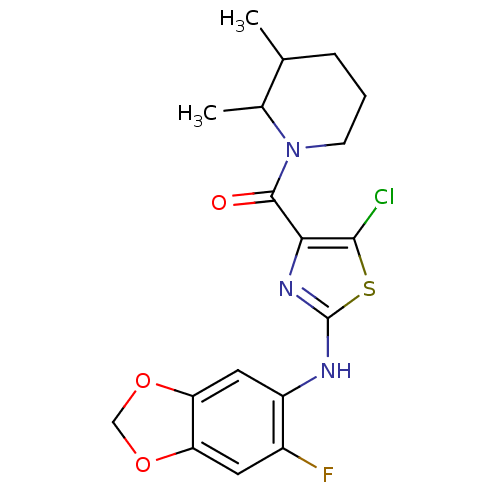
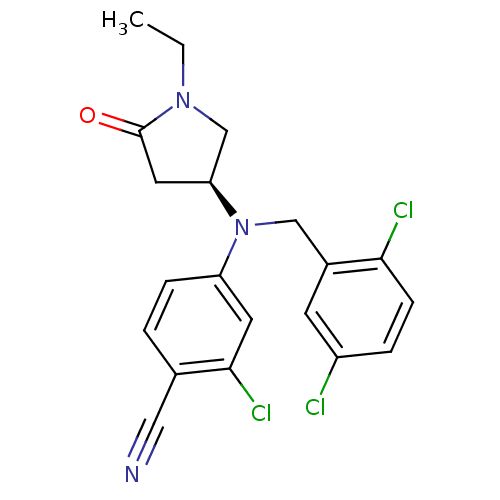
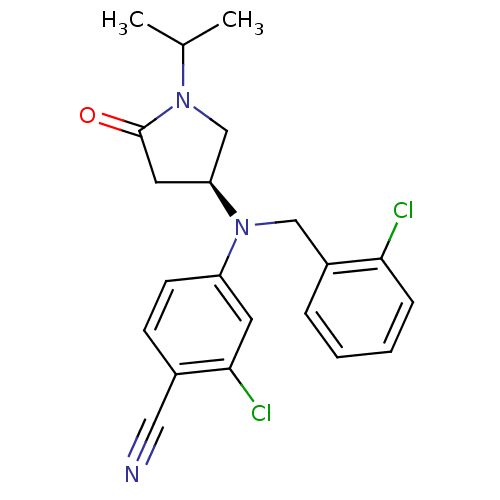
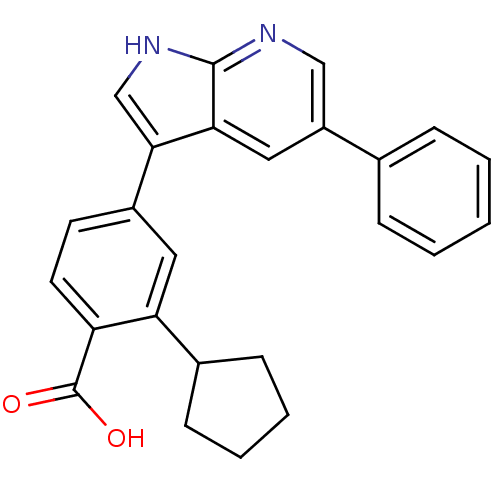
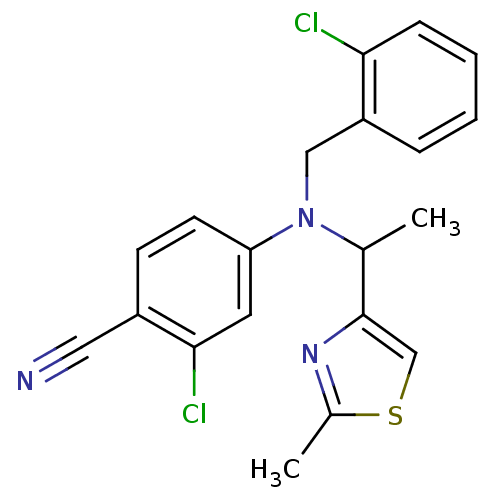
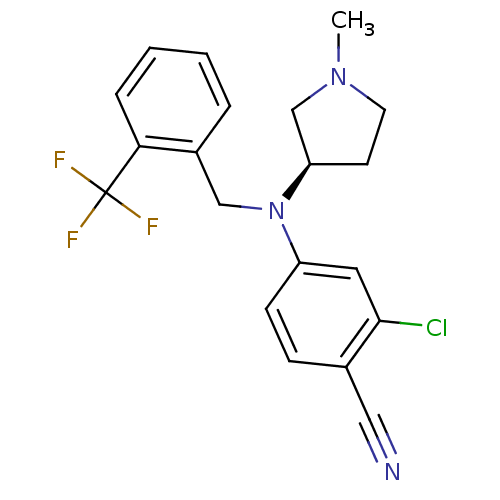
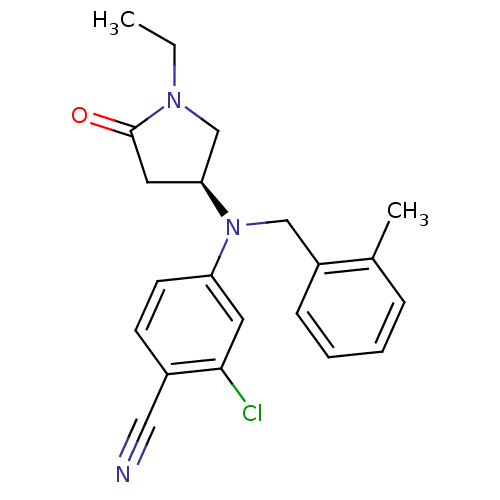
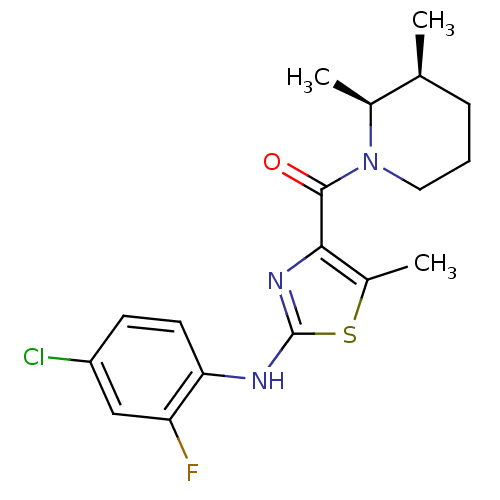
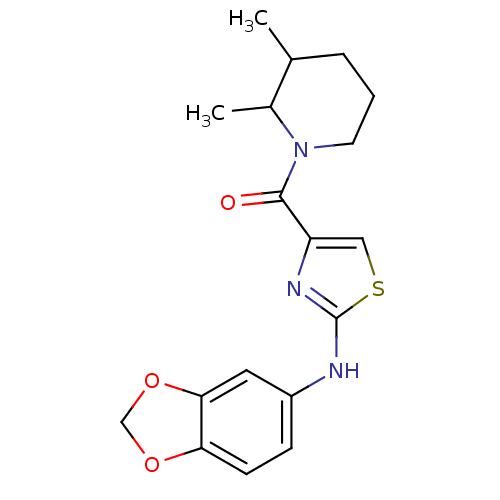
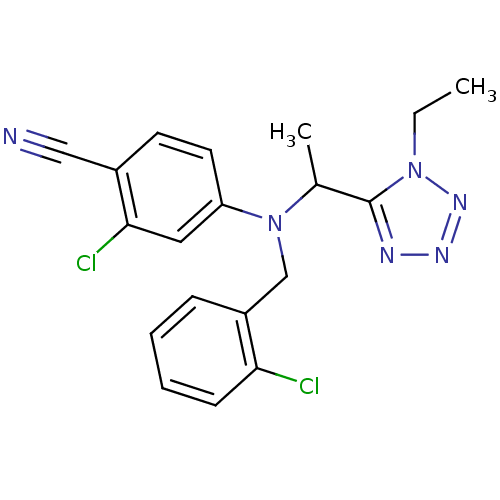
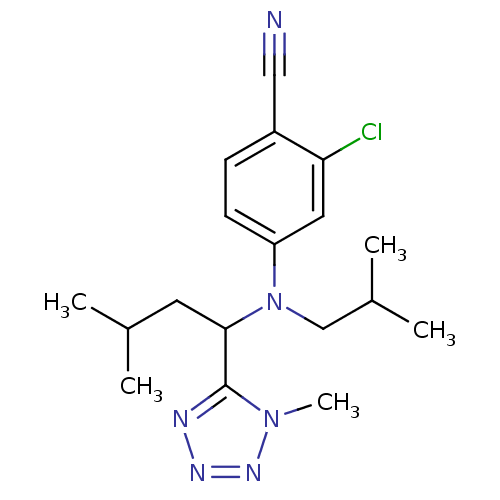
TargetBone morphogenetic protein 1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.00680nMAssay Description:Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs...More data for this Ligand-Target Pair
Affinity DataKi: 0.0310nMAssay Description:Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst...More data for this Ligand-Target Pair
Affinity DataKi: 0.0390nMAssay Description:Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst...More data for this Ligand-Target Pair
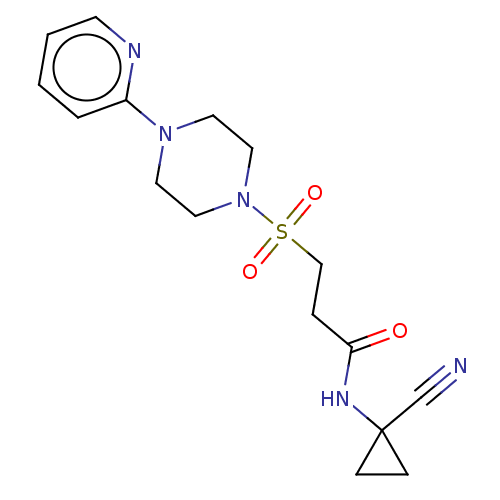
TargetBone morphogenetic protein 1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0400nMAssay Description:Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs...More data for this Ligand-Target Pair
Affinity DataKi: 0.0900nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 0.0900nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 0.240nMAssay Description:Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst...More data for this Ligand-Target Pair
Affinity DataKi: 0.25nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 0.25nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst...More data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 345nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 345nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll...More data for this Ligand-Target Pair
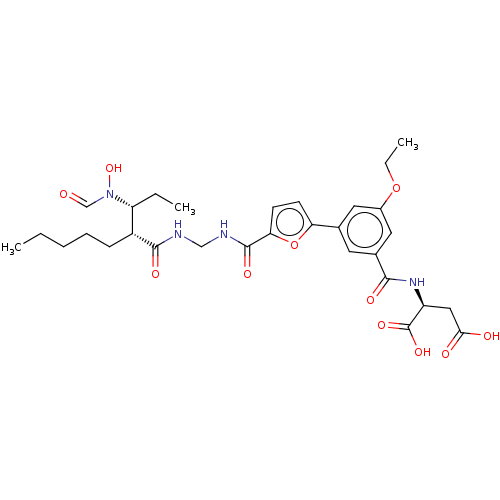
TargetBone morphogenetic protein 1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5...More data for this Ligand-Target Pair
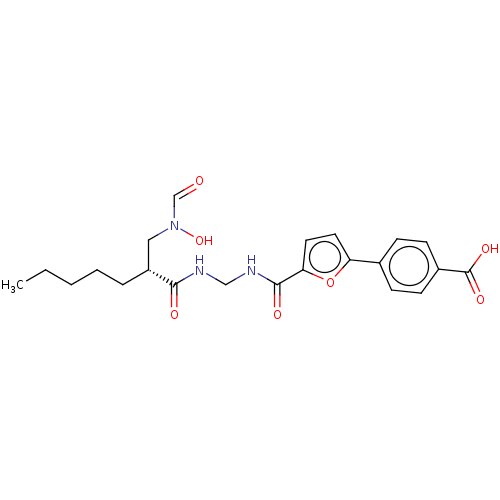
TargetBone morphogenetic protein 1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of GST-tagged PI4K-alpha (1 to 2044) (unknown origin) using D-myo-phosphatidylinositol as substrate preincubated for 30 mins followed by s...More data for this Ligand-Target Pair
TargetBone morphogenetic protein 1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5...More data for this Ligand-Target Pair
TargetBone morphogenetic protein 1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5...More data for this Ligand-Target Pair
TargetBone morphogenetic protein 1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5...More data for this Ligand-Target Pair
TargetBone morphogenetic protein 1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5...More data for this Ligand-Target Pair
TargetBone morphogenetic protein 1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5...More data for this Ligand-Target Pair
TargetBone morphogenetic protein 1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5...More data for this Ligand-Target Pair
TargetShort transient receptor potential channel 6(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBone morphogenetic protein 1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Binding affinity to progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Binding affinity to progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Binding affinity to progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Binding affinity to progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Binding affinity to progesterone receptorMore data for this Ligand-Target Pair
TargetShort transient receptor potential channel 6(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
TargetShort transient receptor potential channel 6(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)