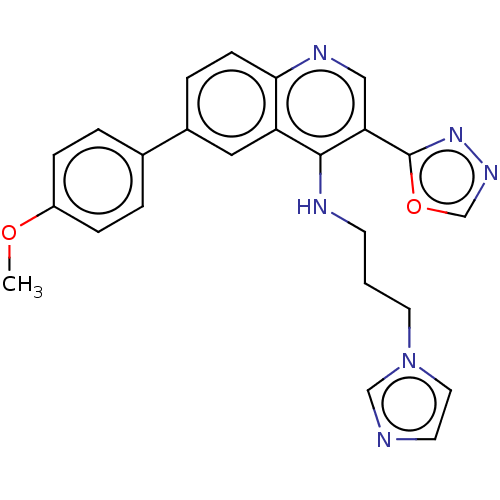
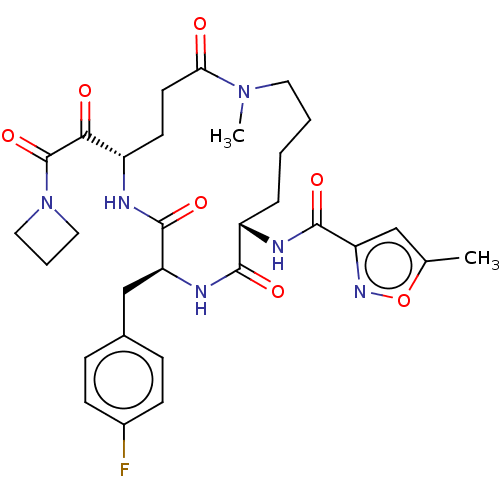
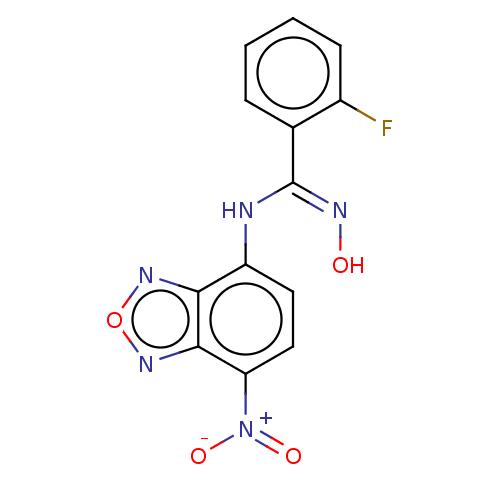
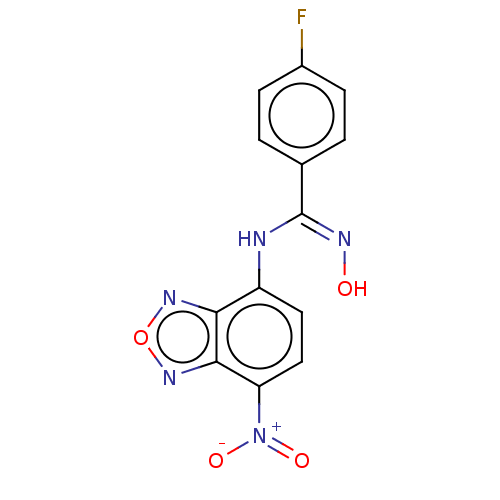
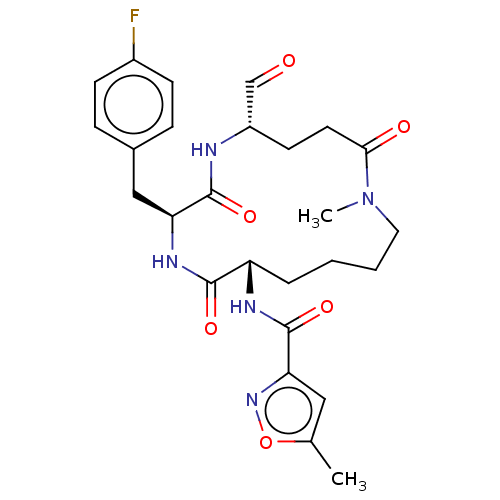
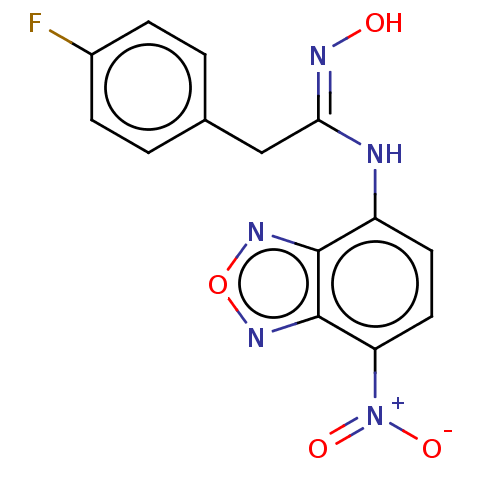
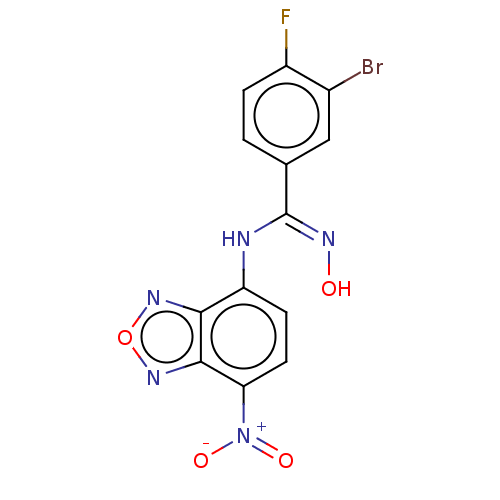
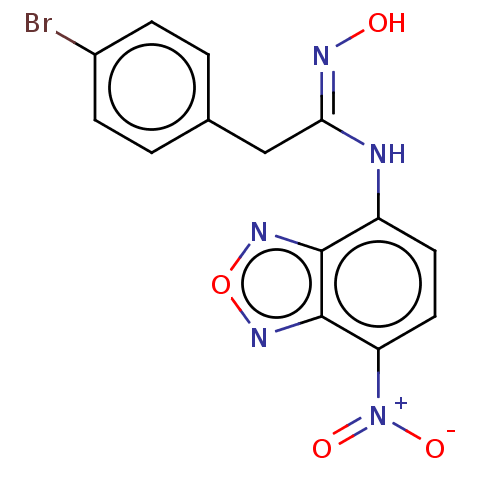
Affinity DataKi: 267nMAssay Description:Uncompetitive inhibition of human thrombin using T1637 as substrate by Michaelis-Menten plot analysisMore data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of human recombinant aromatase expressed in baculovirus-infected cell system using 7-methoxy-trifluoromethylcoumarin as substrate after 30...More data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of aromataseMore data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
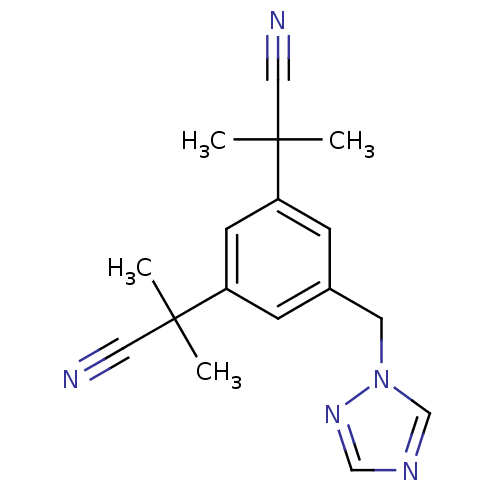
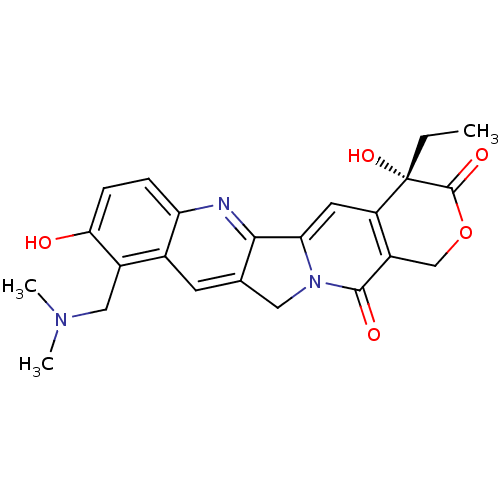
TargetDNA topoisomerase 1(Homo sapiens (Human))
CSIR-Indian Institute of Chemical Biology
Curated by ChEMBL
CSIR-Indian Institute of Chemical Biology
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Poison activity at recombinant human TOP1 expressed in baculovirus infected Sf9 insect cells assessed as decrease in relaxed supercoiled pBS(SK+) DNA...More data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
CSIR-Indian Institute of Chemical Biology
Curated by ChEMBL
CSIR-Indian Institute of Chemical Biology
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Poison activity at recombinant human TOP1 expressed in baculovirus infected Sf9 insect cells assessed as decrease in relaxed supercoiled pBS(SK+) DNA...More data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
CSIR-Indian Institute of Chemical Biology
Curated by ChEMBL
CSIR-Indian Institute of Chemical Biology
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Poison activity at recombinant human TOP1 expressed in baculovirus infected Sf9 insect cells assessed as decrease in relaxed supercoiled pBS(SK+) DNA...More data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 35nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
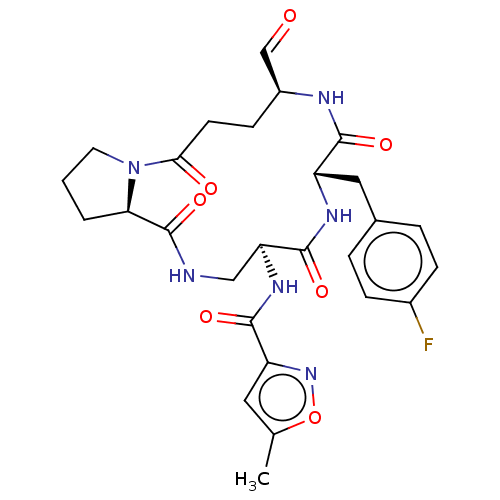
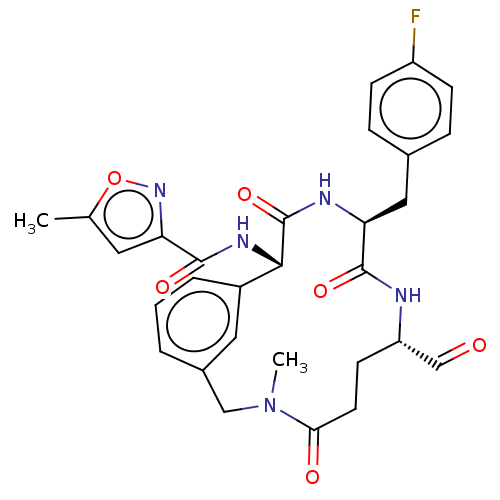
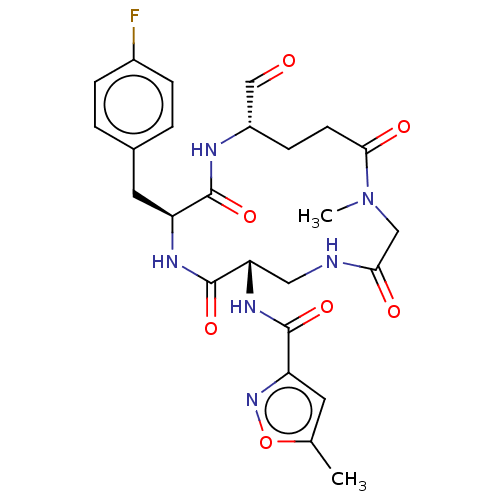
Affinity DataIC50: 39nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 49nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea...More data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 59nMAssay Description:Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea...More data for this Ligand-Target Pair
Affinity DataIC50: 59nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysisMore data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
Affinity DataIC50: 63nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 64nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 66nMAssay Description:Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea...More data for this Ligand-Target Pair
Affinity DataIC50: 71nMAssay Description:Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea...More data for this Ligand-Target Pair
Affinity DataIC50: 73nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 75nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 75nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 78nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 84nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 91nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 92nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 98nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 102nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 105nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 106nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 107nMAssay Description:Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea...More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea...More data for this Ligand-Target Pair
Affinity DataIC50: 112nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 113nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 117nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 125nMAssay Description:Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea...More data for this Ligand-Target Pair
Affinity DataIC50: 127nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 128nMAssay Description:Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph...More data for this Ligand-Target Pair

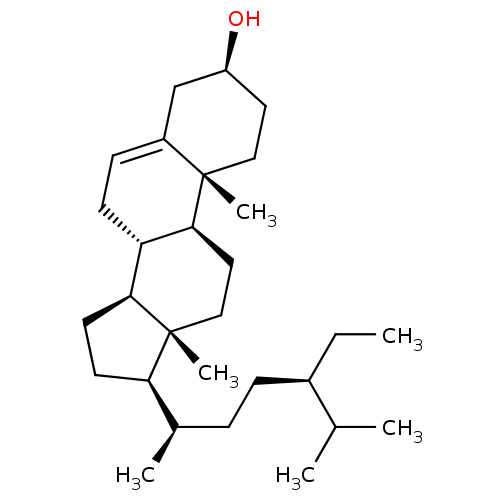

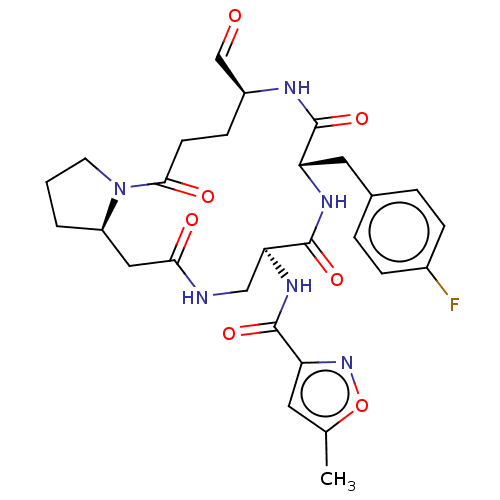
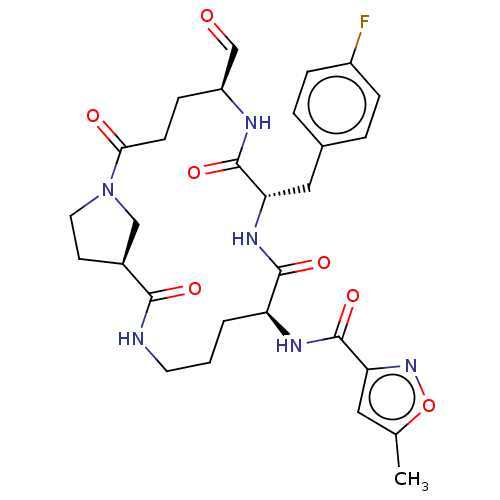

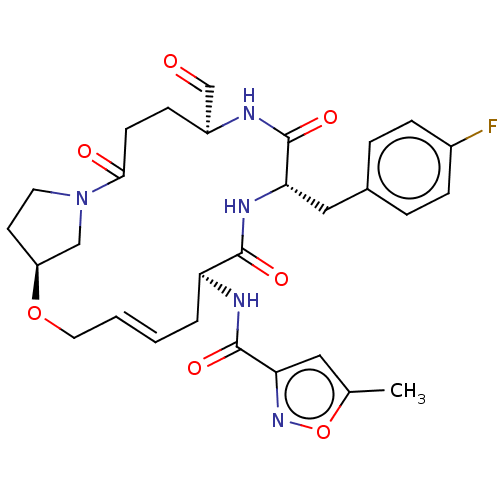

 3D Structure (crystal)
3D Structure (crystal)