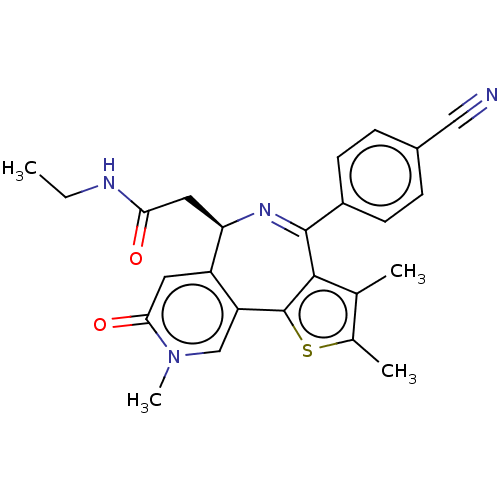
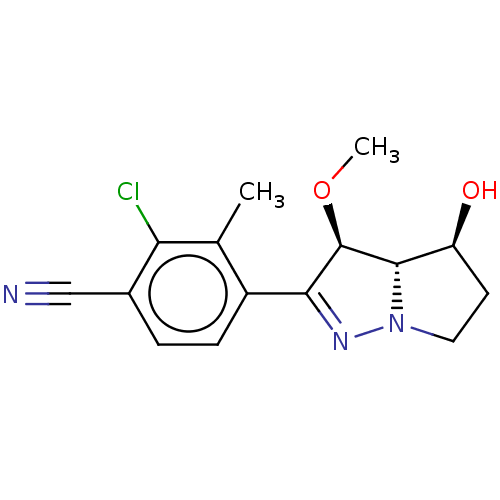
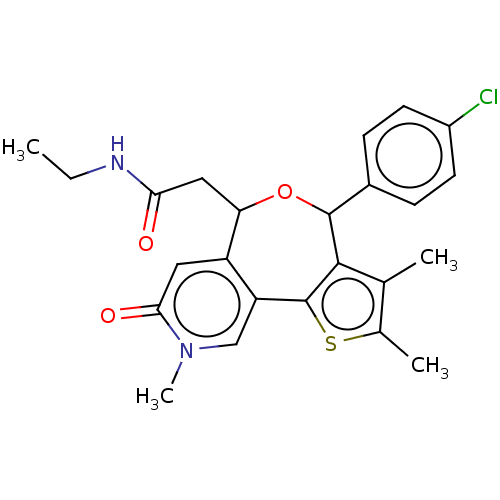
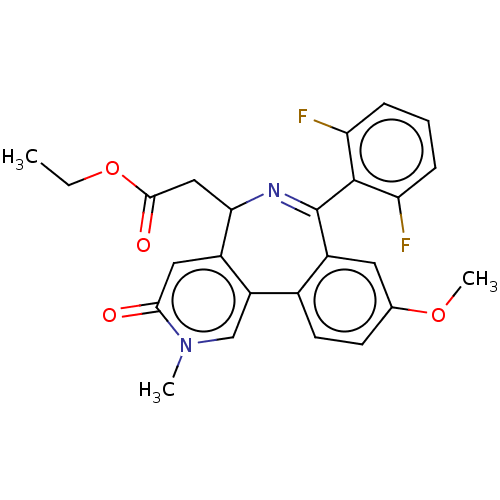
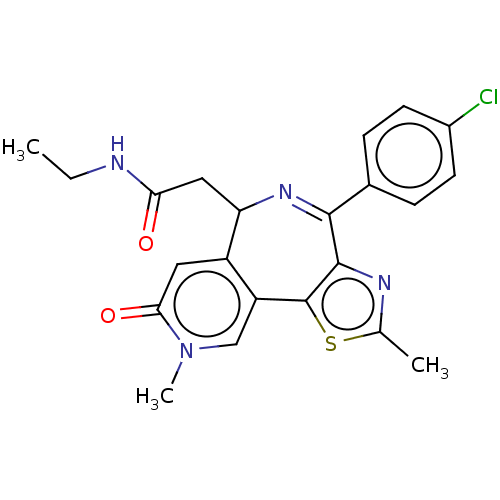
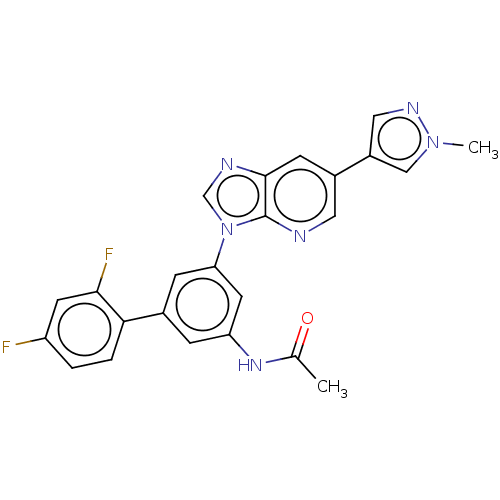
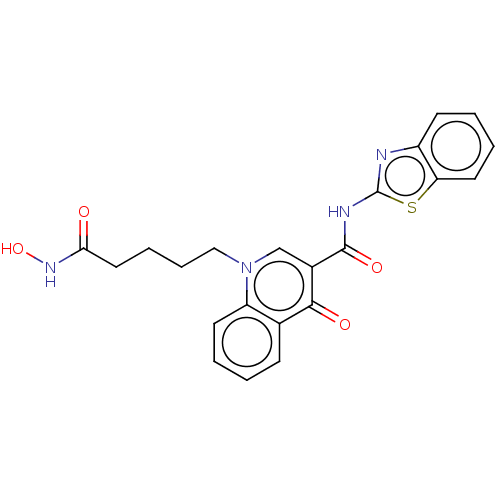
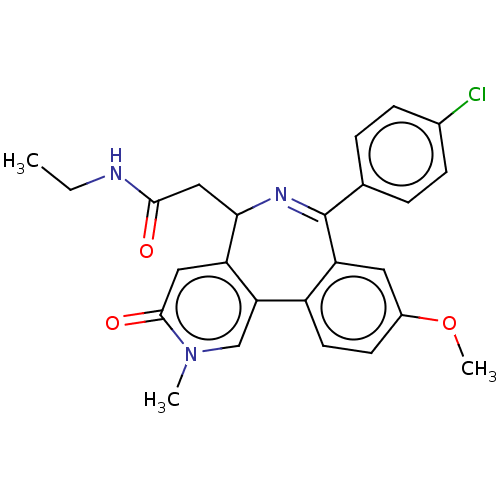
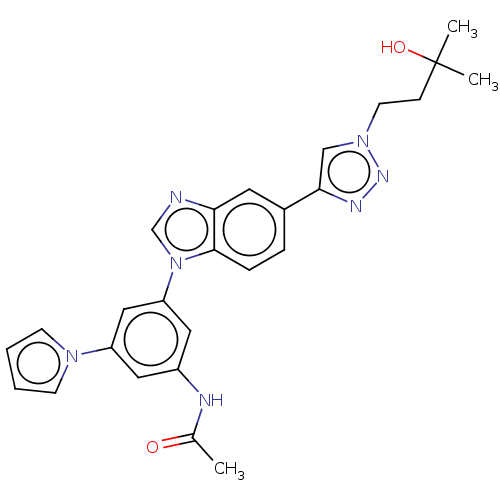
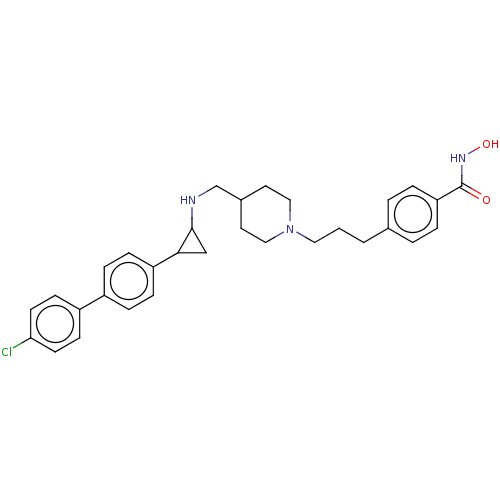
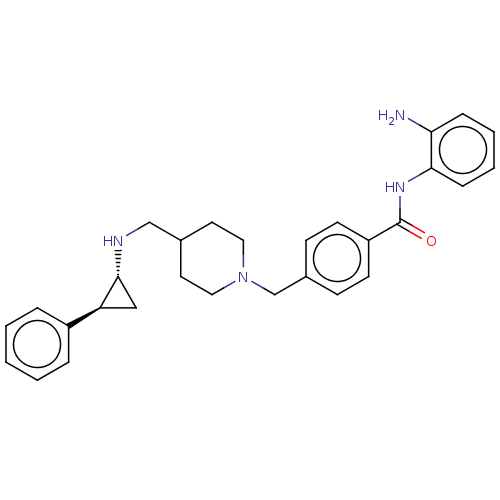
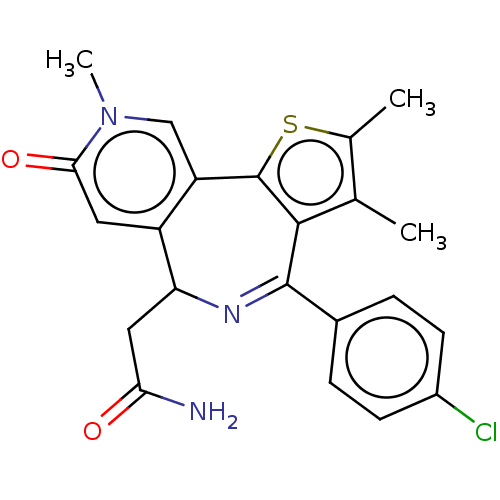
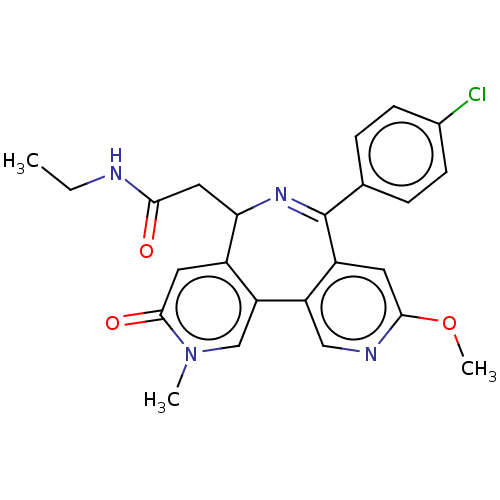
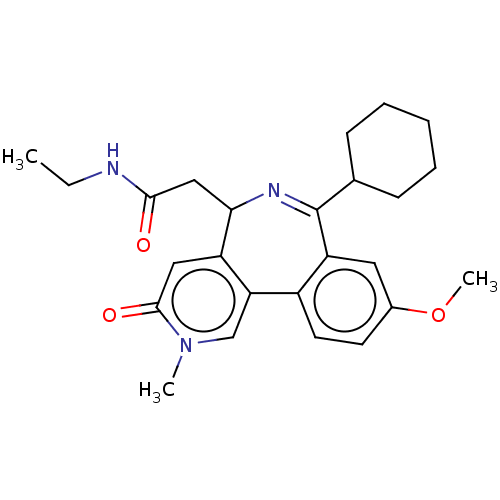
Affinity DataKi: 1nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 38nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 59nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 89nMAssay Description:Displacement of [125I]-apelin-13 from EGFP-fused human APJ receptor delta16 mutant stably expressed in HEK293 cells by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 134nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 147nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 172nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Displacement of [125I]-apelin-13 from EGFP-fused human APJ receptor delta16 mutant stably expressed in HEK293 cells by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 187nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Displacement of [125I]-pE13F from rat EGFP-tagged APJ receptor stably expressed in CHO cell membrane measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Displacement of [125I]-apelin-13 from EGFP-fused human APJ receptor delta16 mutant stably expressed in HEK293 cells by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Displacement of [125I]-pE13F from human APJ receptor stably expressed in CHO cell membrane after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 449nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 571nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 690nMAssay Description:Displacement of [125I]-apelin-13 from EGFP-fused human APJ receptor delta16 mutant stably expressed in HEK293 cells by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMAssay Description:Displacement of [125I]-apelin-13 from human APJ receptor stably expressed in CHOK1 cell membrane measured after 2 hrs by topcount scintillation count...More data for this Ligand-Target Pair
TargetHistone deacetylase 1/11/4/6/8(Homo sapiens (Human))
Orchid Chemicals & Pharmaceuticals Limited
Orchid Chemicals & Pharmaceuticals Limited
Affinity DataIC50: 0.100nMAssay Description:Histone Deacetylase (HDAC) Inhibition Assay using Boc-Lys(Ac)-AMC Substrate: Inhibition of HDAC has been implicated to modulate transcription and to ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
TargetAndrogen receptor(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Displacement of [3H]DHT from androgen receptor in human MDA-MB-435 cells after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Compounds were screened in the TR-FRET assay with FGFR1 kinase. 5 ng of FGFR1 [Upstate, USA] kinase was used for assay. The compound was incubated wi...More data for this Ligand-Target Pair
TargetHistone deacetylase 1/11/4/6/8(Homo sapiens (Human))
Orchid Chemicals & Pharmaceuticals Limited
Orchid Chemicals & Pharmaceuticals Limited
Affinity DataIC50: 1.5nMAssay Description:Histone Deacetylase (HDAC) Inhibition Assay using Boc-Lys(Ac)-AMC Substrate: Inhibition of HDAC has been implicated to modulate transcription and to ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Compounds were screened in the TR-FRET assay with FGFR1 kinase. 5 ng of FGFR1 [Upstate, USA] kinase was used for assay. The compound was incubated wi...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:LSD1 enzyme was produced in house. Tranylcypromine (TCP), LSD1 inhibitor was procured from Selleckchem. LSD1 enzyme, TCP and Biotinylated peptide sub...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:LSD1 enzyme was produced in house. Tranylcypromine (TCP), LSD1 inhibitor was procured from Selleckchem. LSD1 enzyme, TCP and Biotinylated peptide sub...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:LSD1 enzyme was produced in house. Tranylcypromine (TCP), LSD1 inhibitor was procured from Selleckchem. LSD1 enzyme, TCP and Biotinylated peptide sub...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:LSD1 enzyme was produced in house. Tranylcypromine (TCP), LSD1 inhibitor was procured from Selleckchem. LSD1 enzyme, TCP and Biotinylated peptide sub...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair

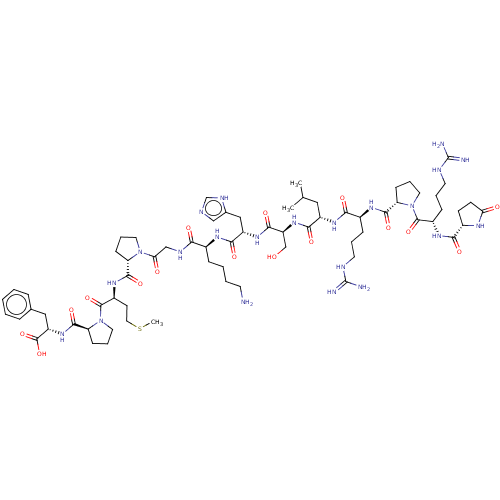
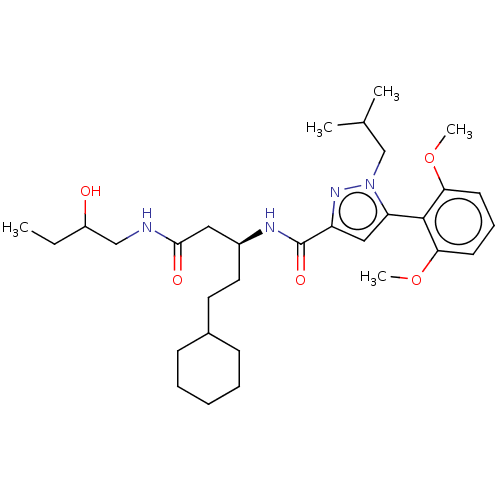


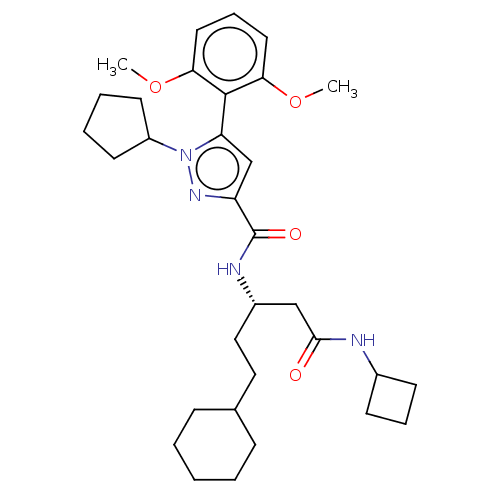

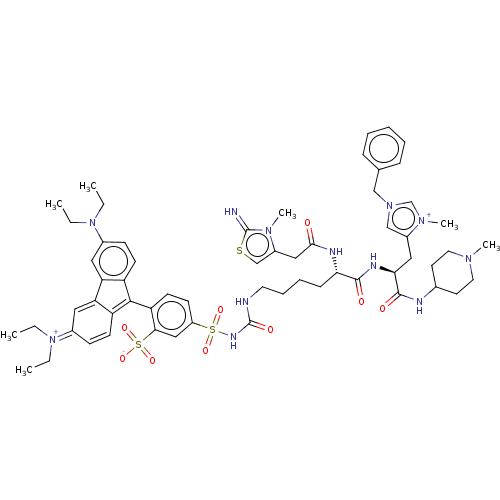
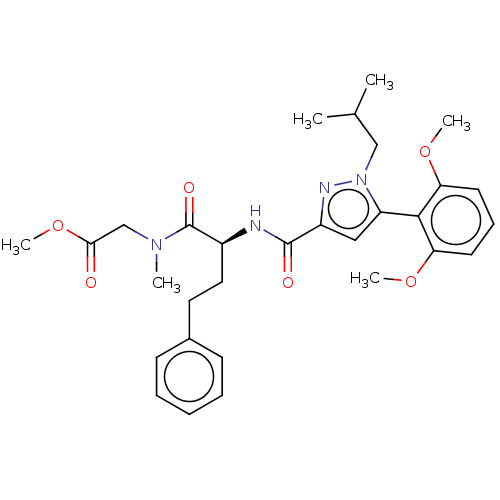


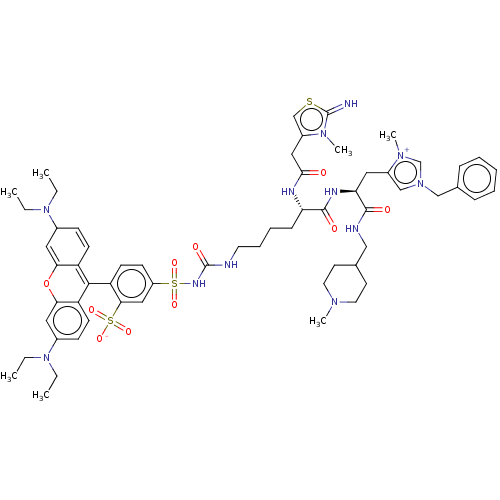

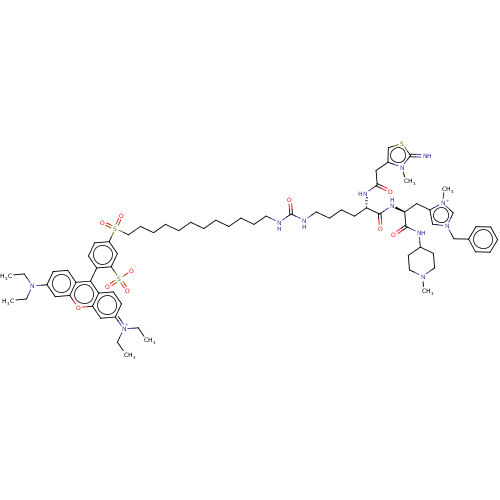


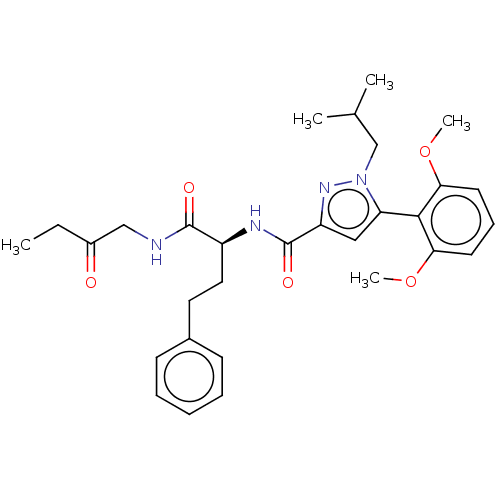
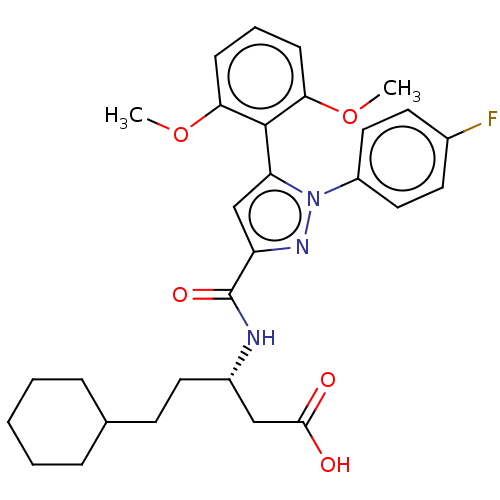
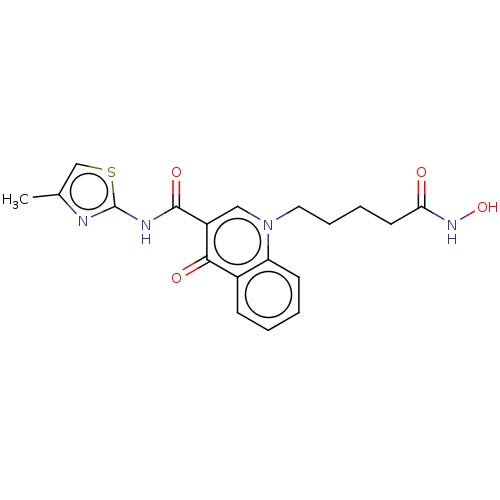
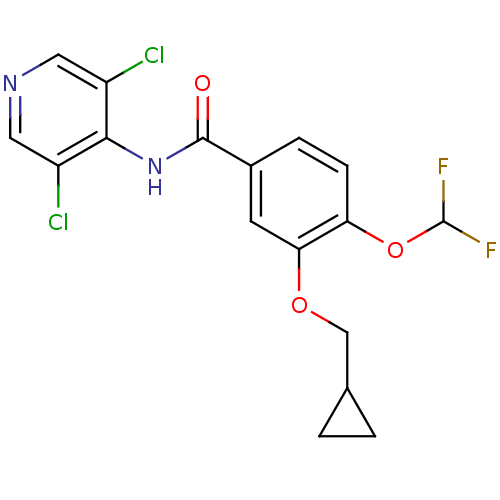
 3D Structure (crystal)
3D Structure (crystal)