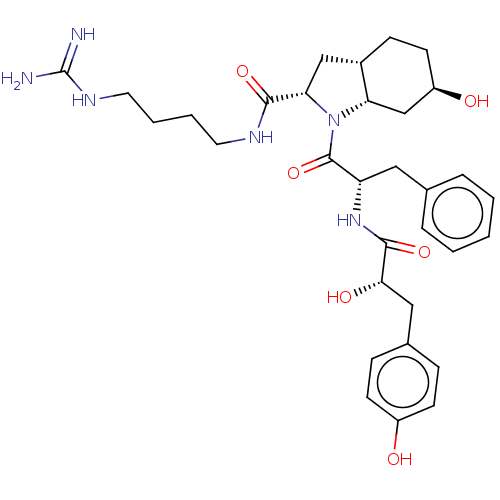
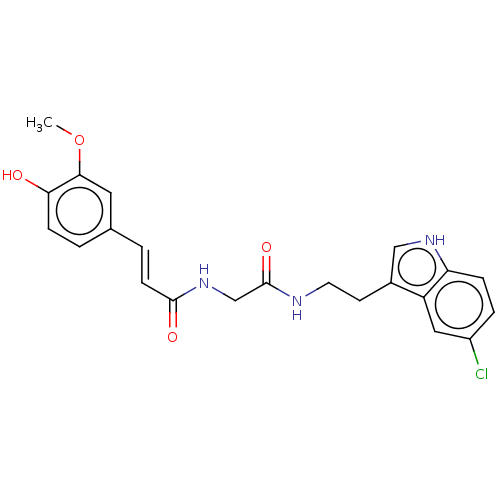
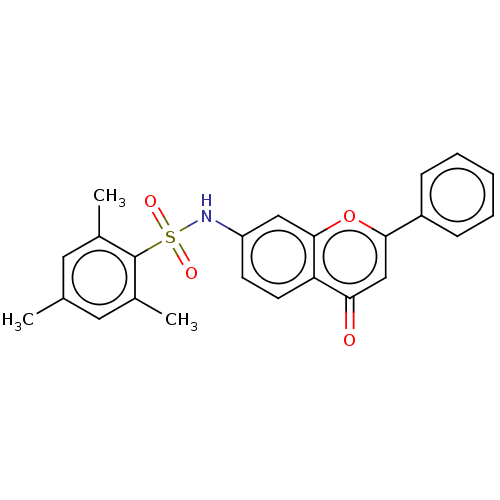
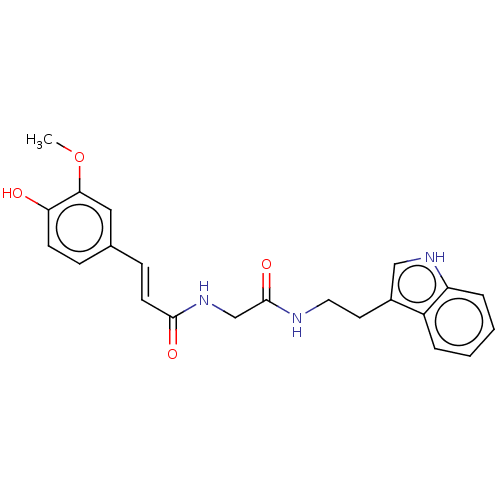
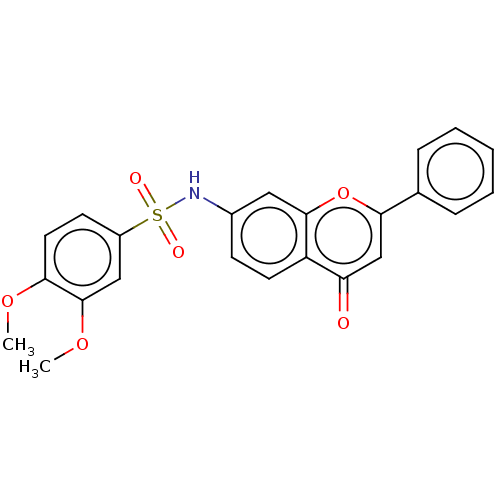
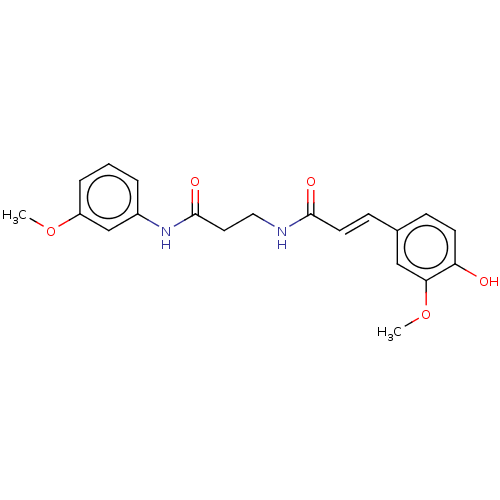
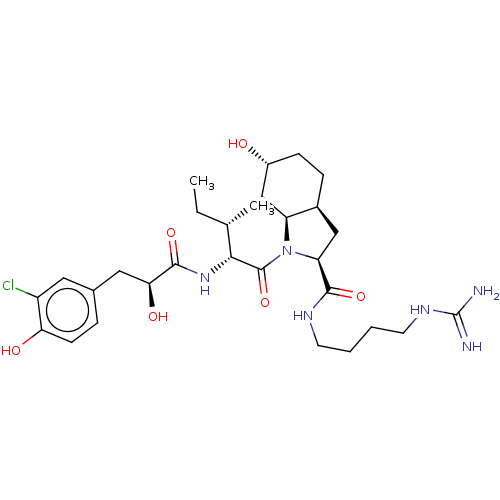
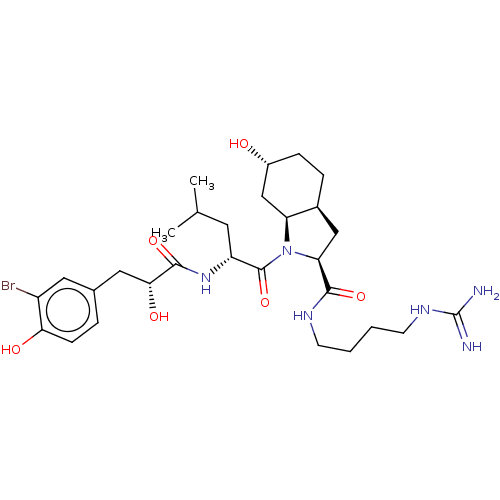
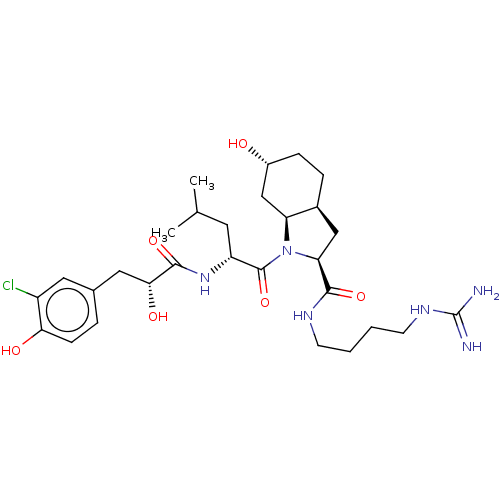
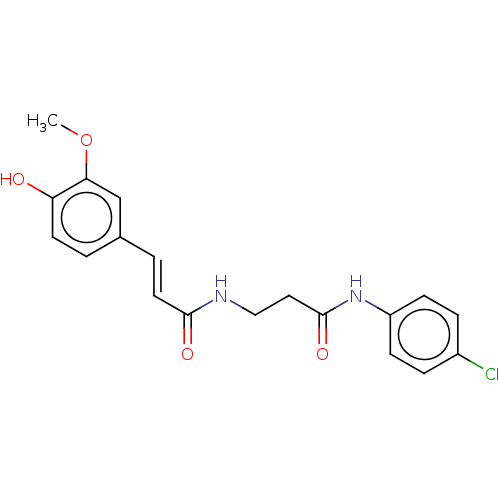
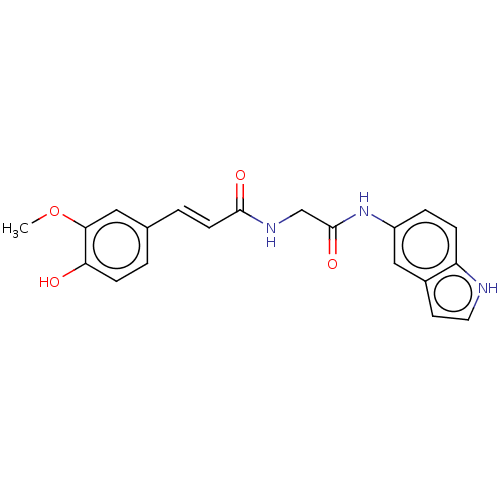
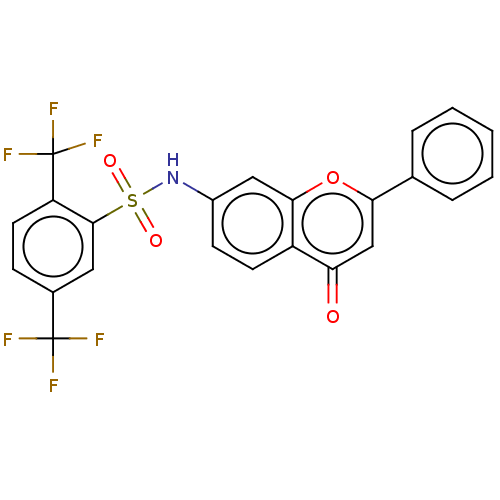
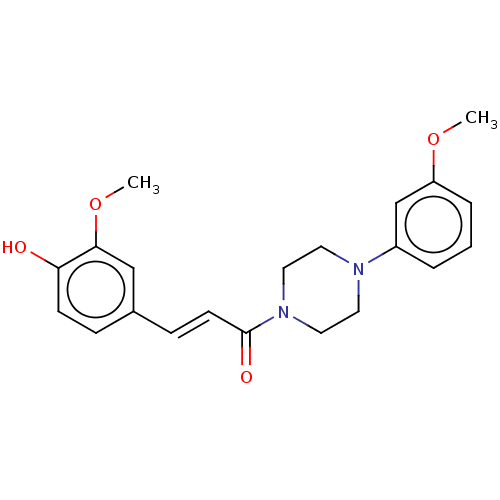
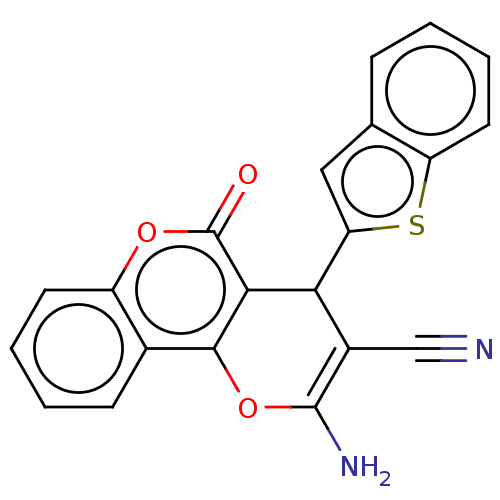
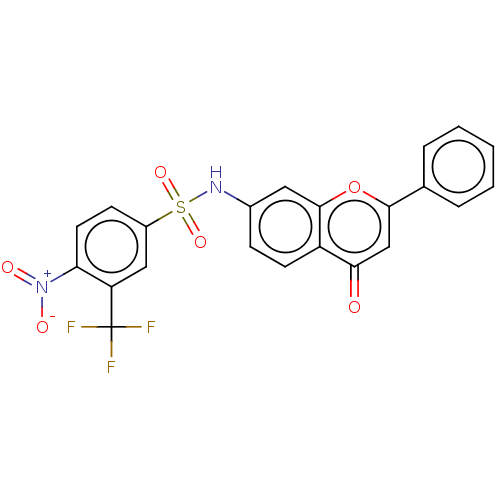
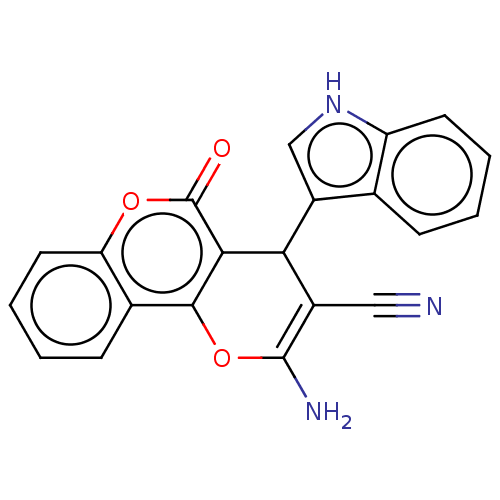
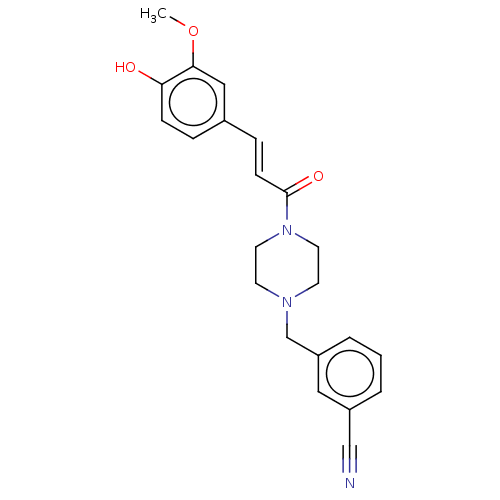
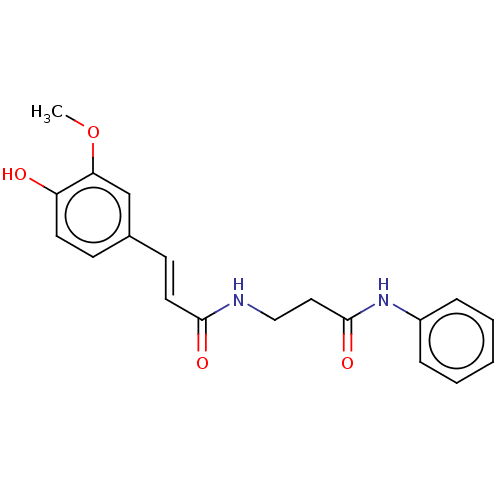
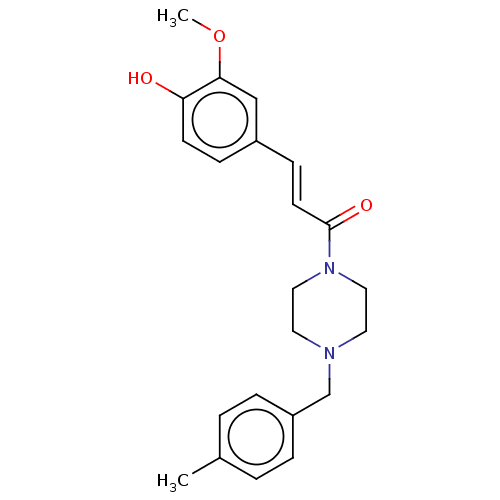
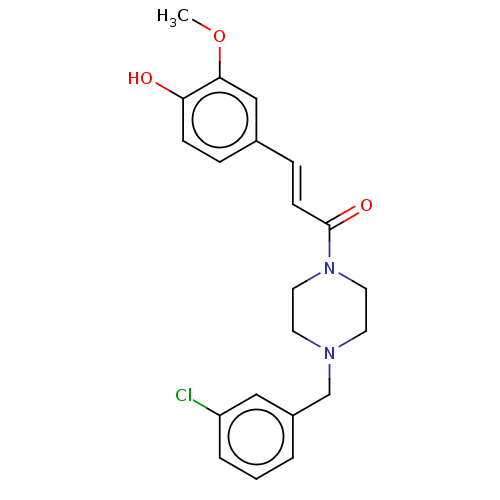
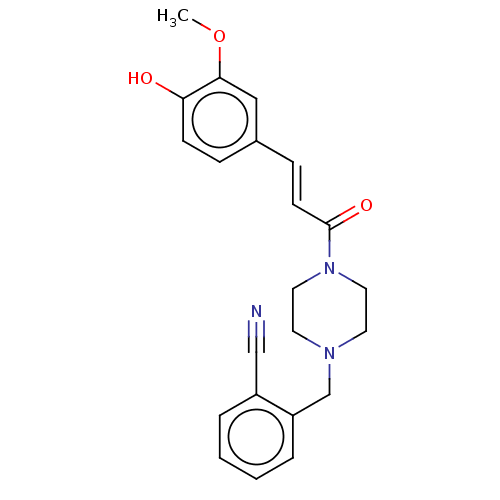
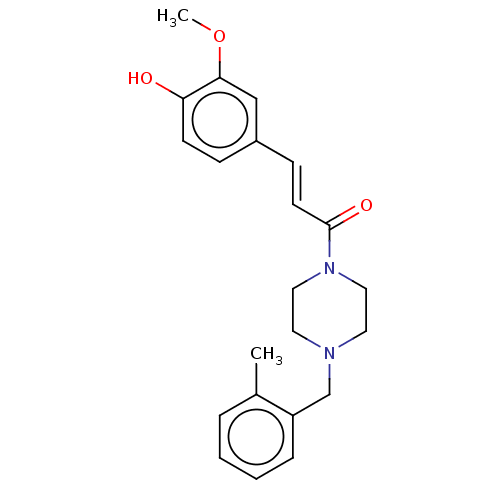
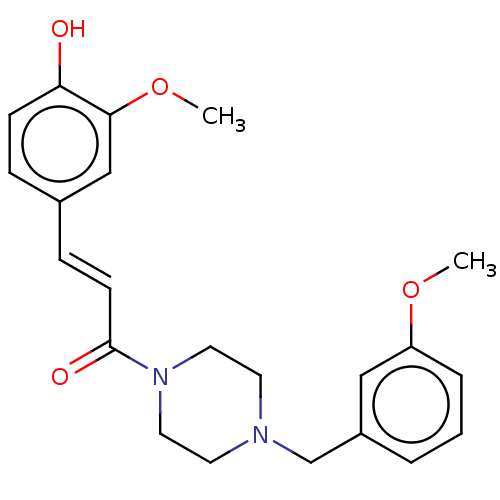
Affinity DataIC50: 60nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 821nMAssay Description:Inhibition of trypsin (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 840nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Affinity DataIC50: 940nMAssay Description:Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro...More data for this Ligand-Target Pair
Affinity DataIC50: 960nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Affinity DataIC50: 980nMAssay Description:Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Affinity DataIC50: 990nMAssay Description:Inhibition of human topoisomerase-2 alpha in human A498 cells assessed as decrease in relaxation of supercoiled DNA at 10 uMMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Displacement of [125I]gp-120 from human CCR5 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.23E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.29E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.42E+3nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Affinity DataIC50: 1.77E+3nMAssay Description:Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro...More data for this Ligand-Target Pair
Affinity DataIC50: 2.16E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.41E+3nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of trypsin (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.14E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of trypsin (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of trypsin (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.32E+3nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.74E+3nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 7.13E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Affinity DataIC50: 8.15E+3nMAssay Description:Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro...More data for this Ligand-Target Pair
Affinity DataIC50: 9.91E+3nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Affinity DataIC50: 1.03E+4nMAssay Description:Inhibition of human topoisomerase-2 alpha in human A498 cells assessed as decrease in relaxation of supercoiled DNA at 10 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 1.13E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Affinity DataIC50: 1.21E+4nMAssay Description:Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
Affinity DataIC50: 1.24E+4nMAssay Description:Inhibition of human topoisomerase-2 alpha in human A498 cells assessed as decrease in relaxation of supercoiled DNA at 10 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 1.31E+4nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+4nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.41E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.45E+4nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 1.46E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.47E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.47E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.48E+4nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 1.48E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.48E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.49E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.49E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.52E+4nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair


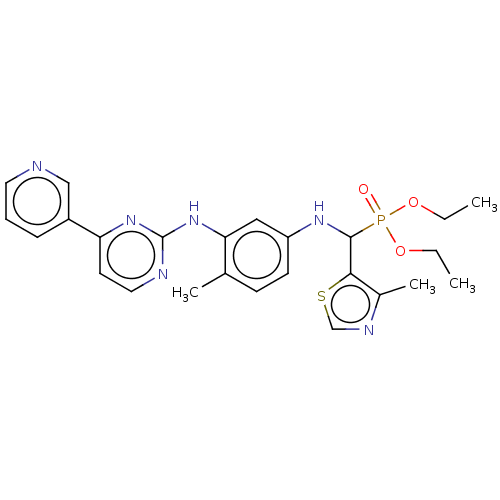


 3D Structure (crystal)
3D Structure (crystal)