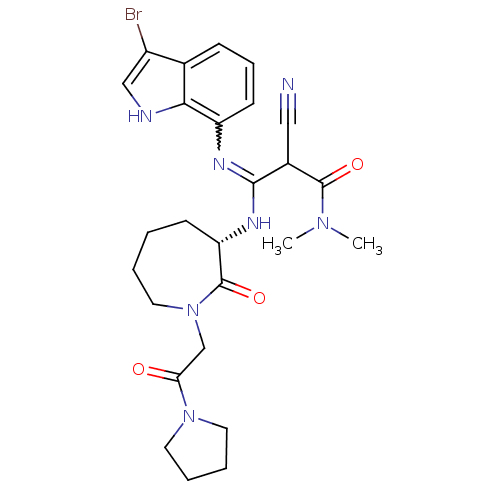
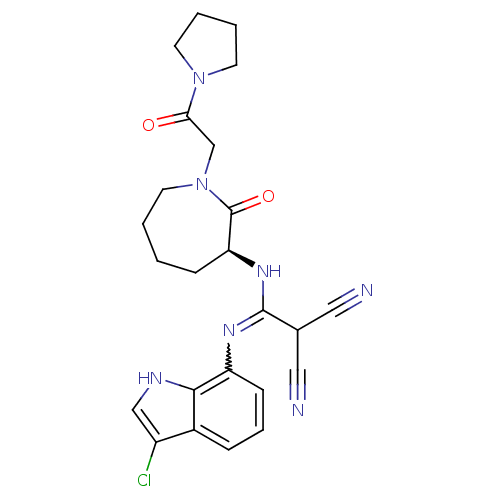
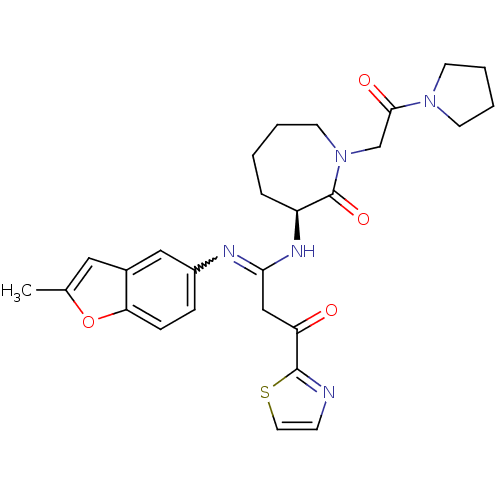
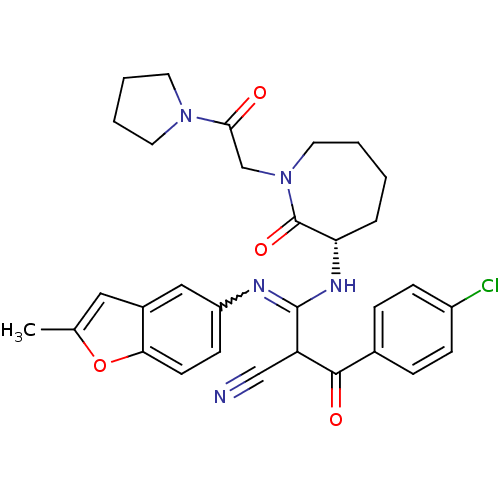
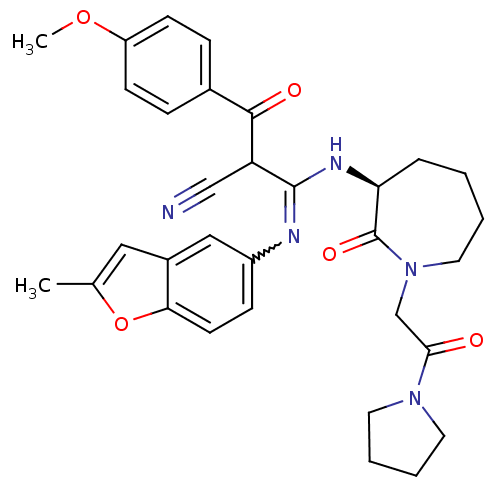
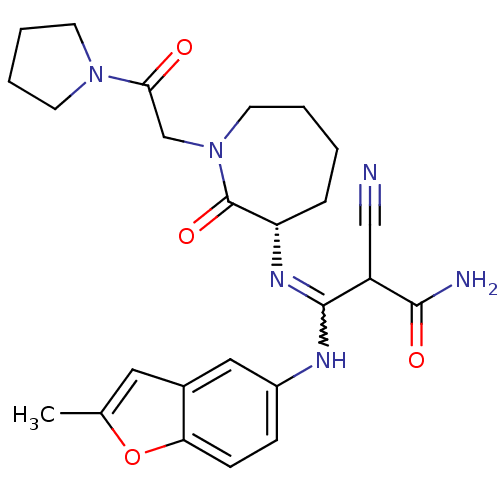
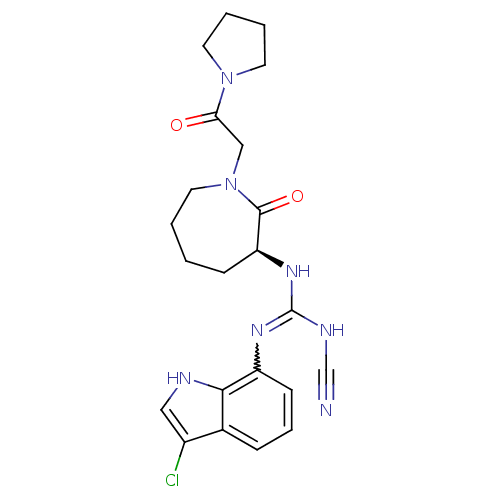
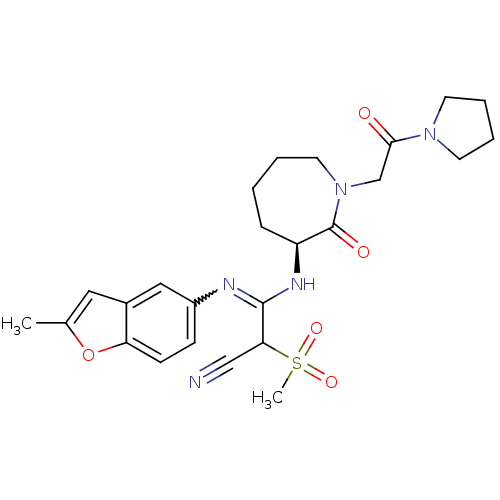
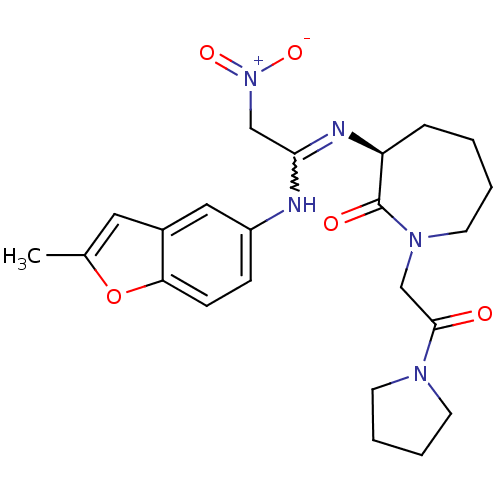
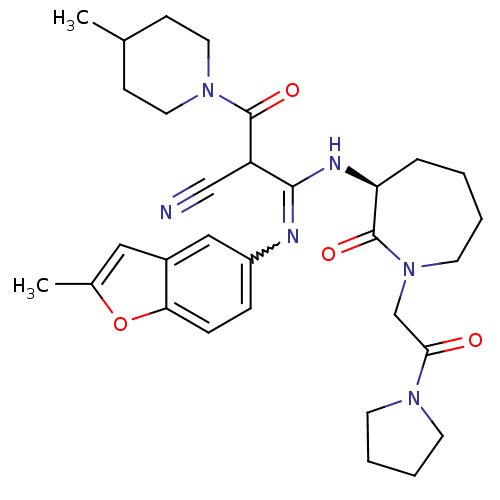
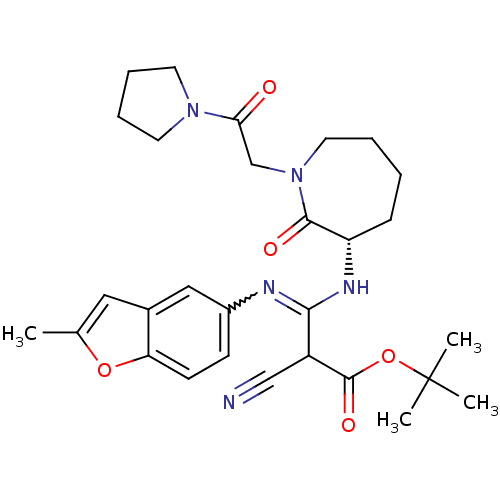
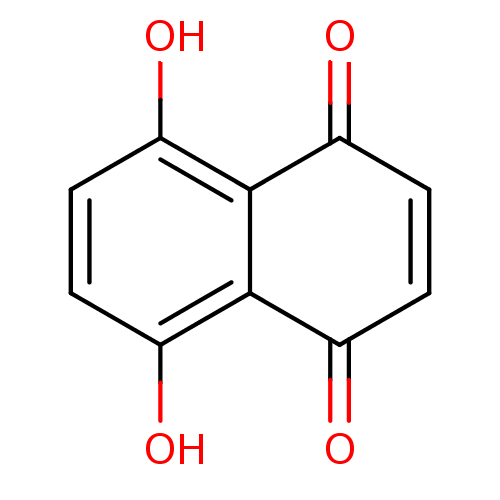
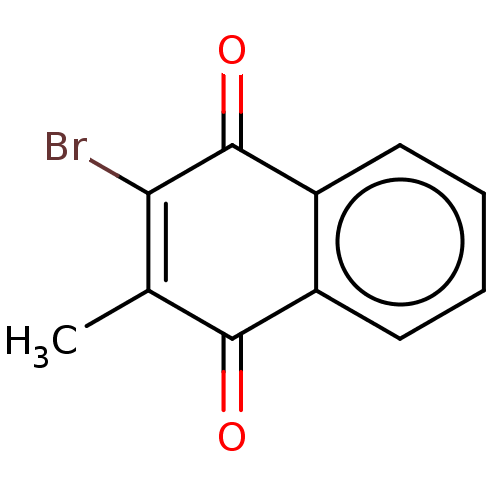
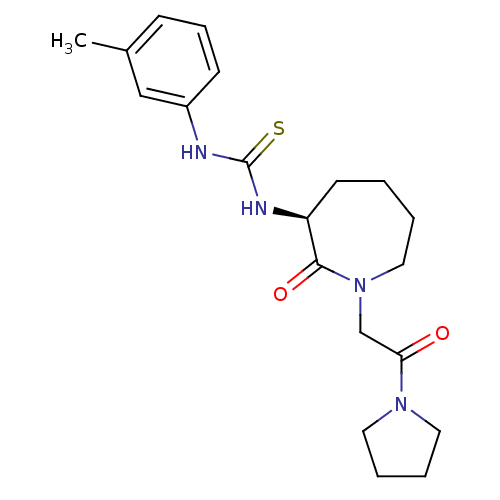
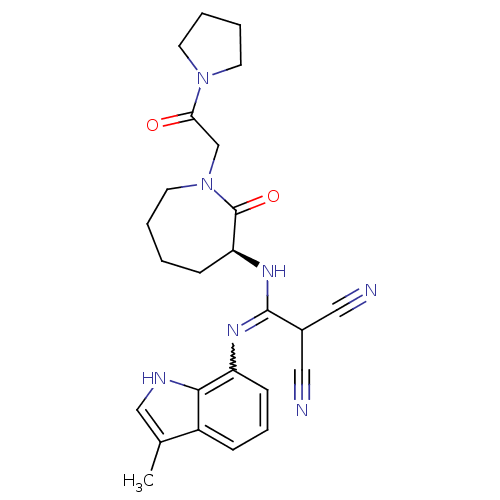
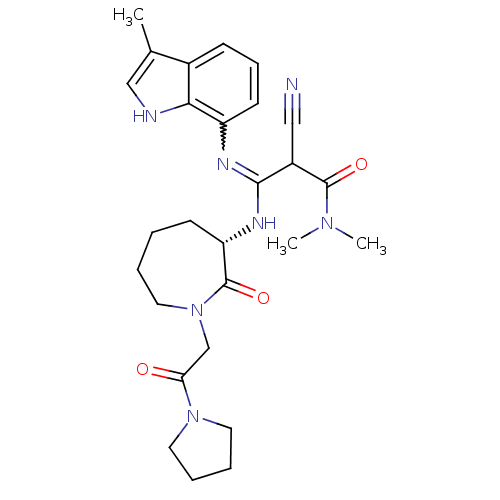
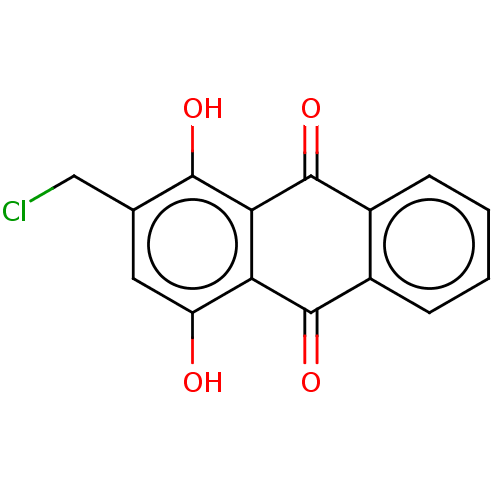
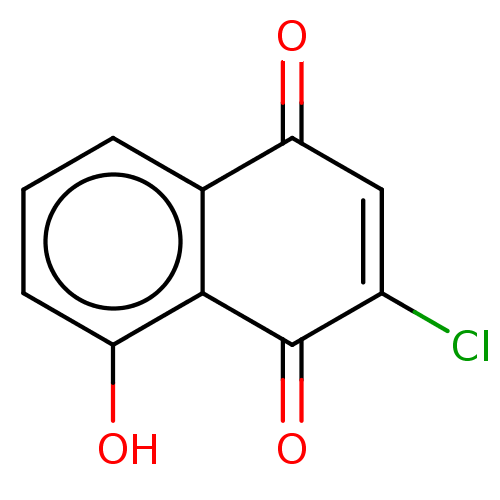
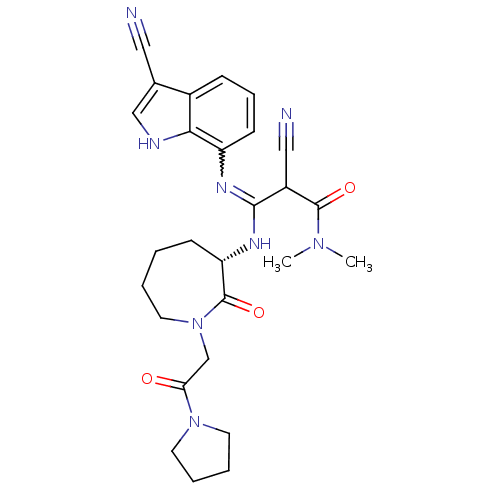
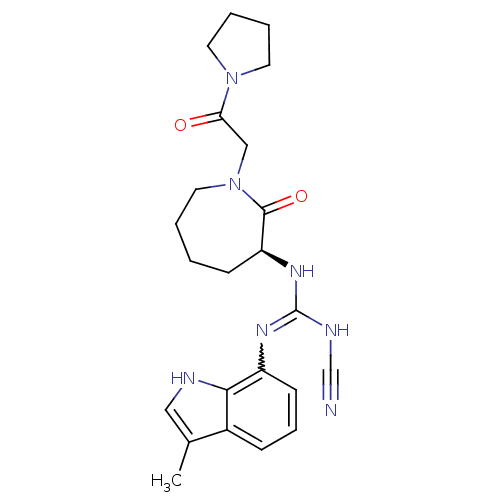
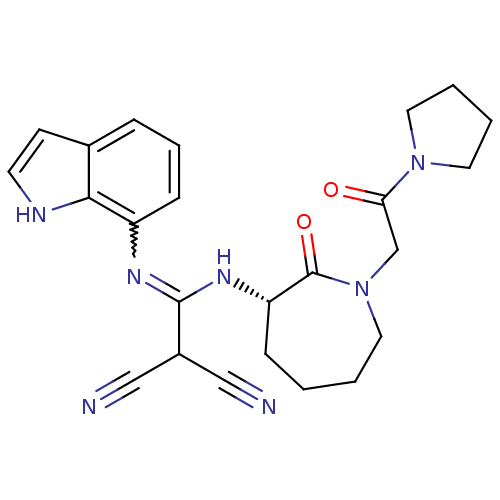
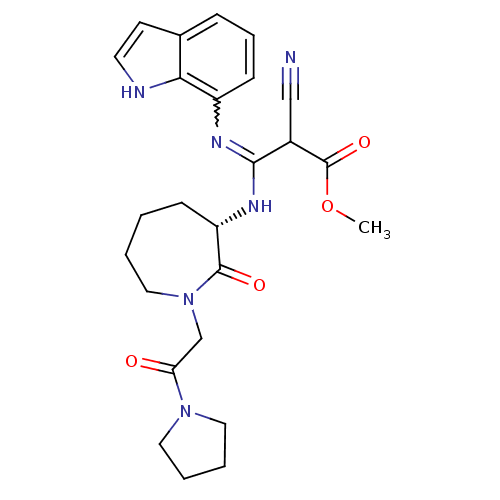
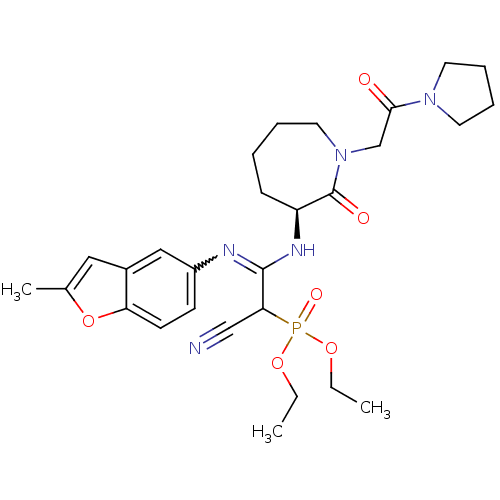
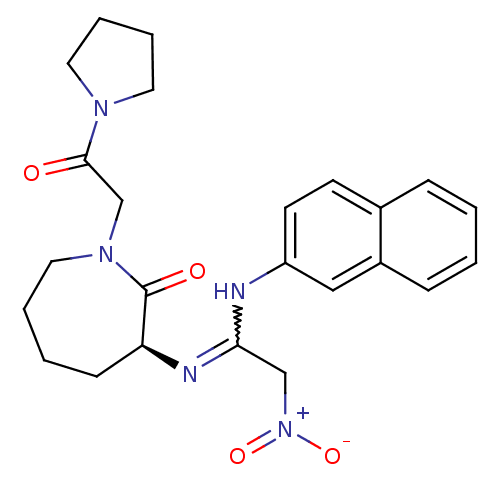
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 6.30E+3nMAssay Description:Binding affinity to thrombinMore data for this Ligand-Target Pair
TargetChymotrypsinogen B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 9.42E+3nMAssay Description:Binding affinity to chymotrypsinMore data for this Ligand-Target Pair
TargetCoagulation factor IX(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to factor IXaMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >1.40E+4nMAssay Description:Binding affinity to urokinaseMore data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Binding affinity to tPAMore data for this Ligand-Target Pair
TargetPlasma kallikrein(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >1.94E+4nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
TargetVitamin K-dependent protein C(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >2.15E+4nMAssay Description:Binding affinity to activated protein CMore data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >2.20E+4nMAssay Description:Binding affinity to plasminMore data for this Ligand-Target Pair
TargetCoagulation factor VII(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: >5.50E+4nMAssay Description:Binding affinity to factor VIIaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.40nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.90nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 9nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 9.30nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 30nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 46nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 51nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 57nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 57nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 110nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 114nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 118nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 290nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 336nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 430nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 539nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 647nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 650nMpH: 7.4 T: 2°CAssay Description:Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 658nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity against human FXaMore data for this Ligand-Target Pair

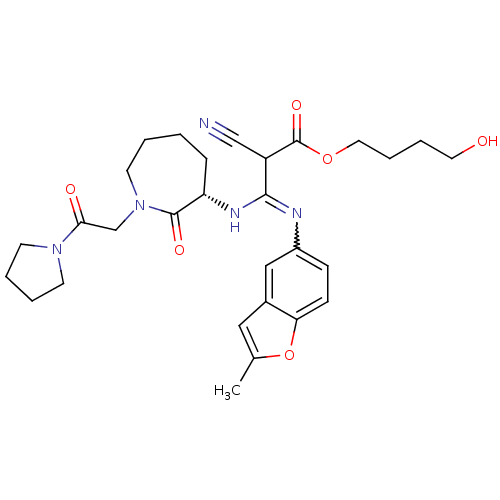
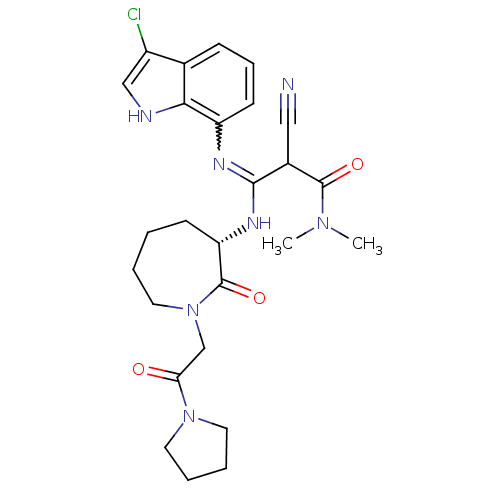
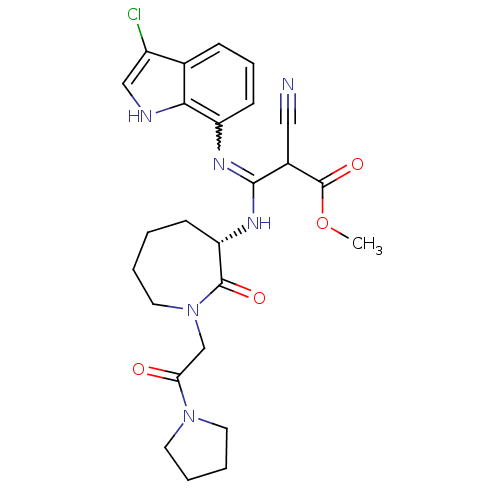
 3D Structure (crystal)
3D Structure (crystal)