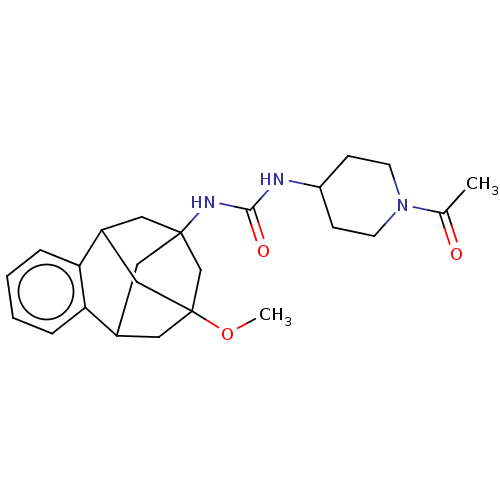
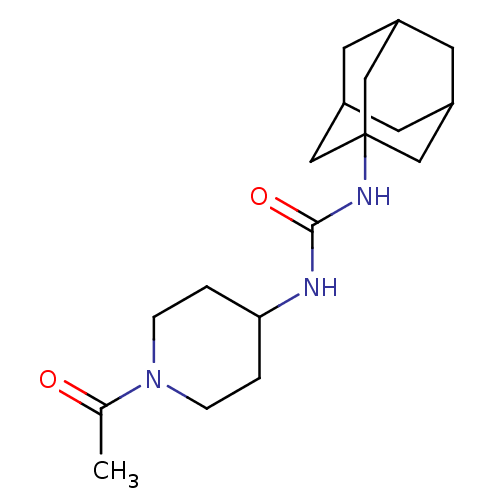
TargetMucosa-associated lymphoid tissue lymphoma translocation protein 1(Homo sapiens (Human))
KU Leuven
Curated by ChEMBL
KU Leuven
Curated by ChEMBL
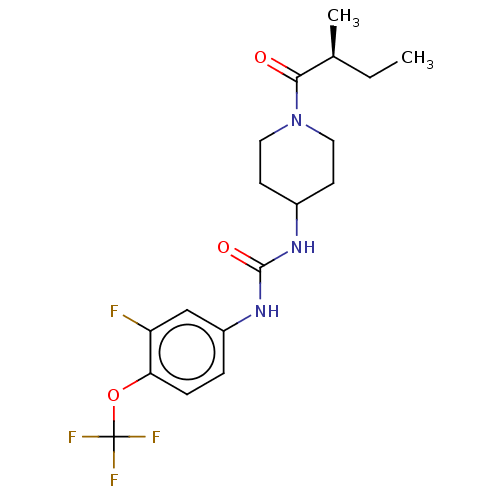
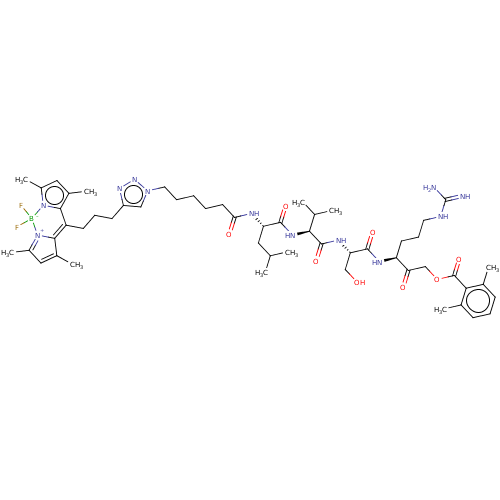
Affinity DataKi: 170nMAssay Description:Inhibition of full length wild type human N-terminal GST-tagged MALT1 catalytic domain (325 to 760 residues) expressed in Escherichia coli BL21 (DE3)...More data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:Inhibition of recombinant human cathepsin B expressed in Escherichia coli BL21 (DE3) using zRR-AMC as substrate measured after 2 hrs by fluorescence ...More data for this Ligand-Target Pair
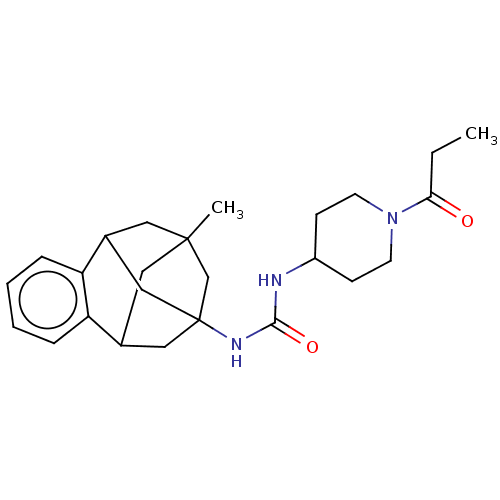
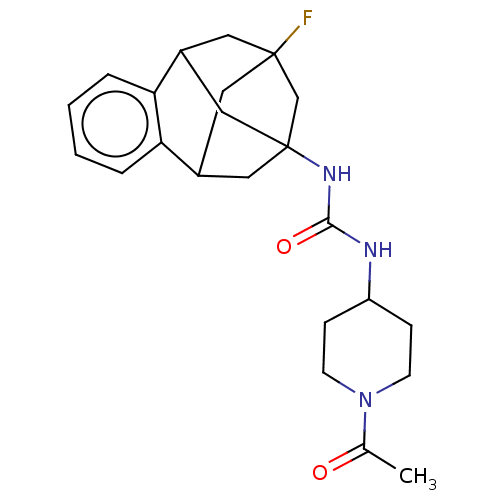
TargetMucosa-associated lymphoid tissue lymphoma translocation protein 1(Homo sapiens (Human))
KU Leuven
Curated by ChEMBL
KU Leuven
Curated by ChEMBL
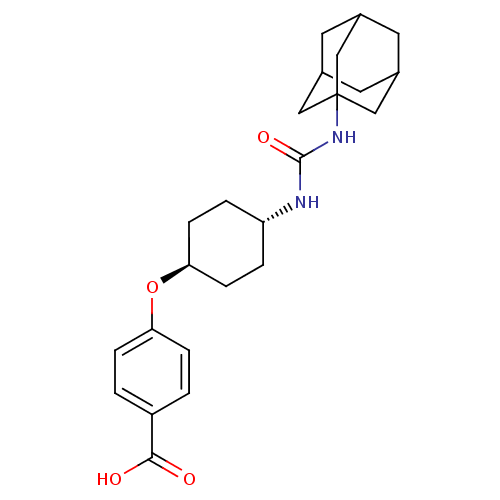
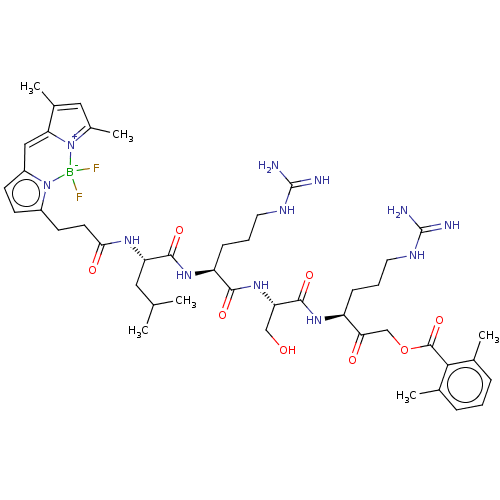
Affinity DataKi: 210nMAssay Description:Inhibition of full length wild type human N-terminal GST-tagged MALT1 catalytic domain (325 to 760 residues) expressed in Escherichia coli BL21 (DE3)...More data for this Ligand-Target Pair
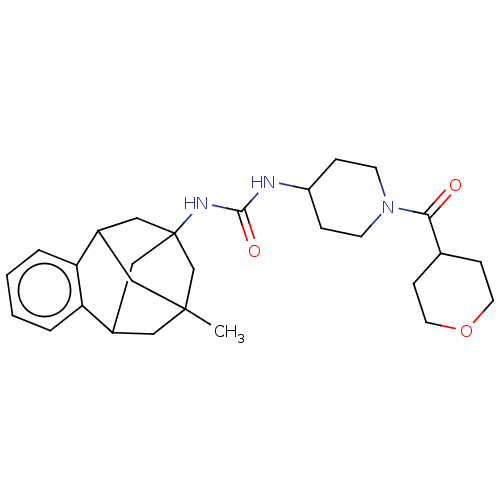
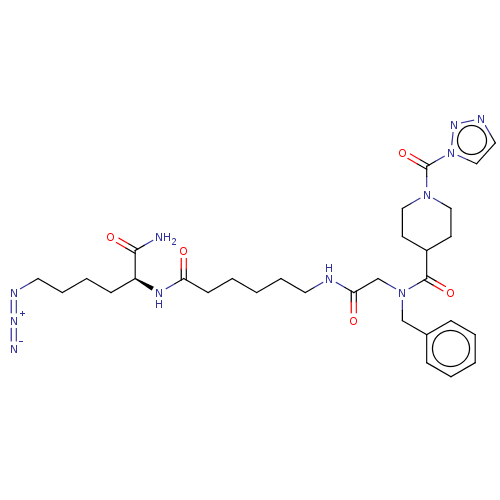
TargetMucosa-associated lymphoid tissue lymphoma translocation protein 1(Homo sapiens (Human))
KU Leuven
Curated by ChEMBL
KU Leuven
Curated by ChEMBL
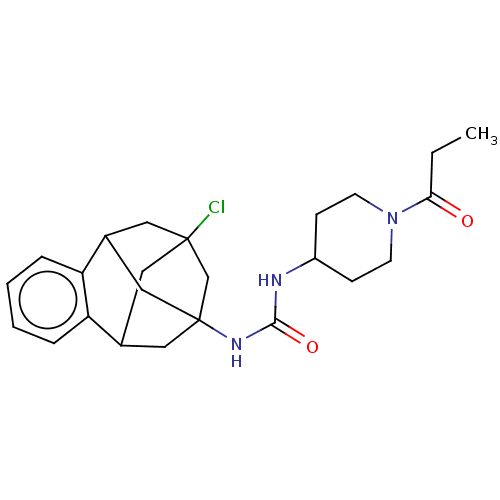
Affinity DataKi: 340nMAssay Description:Inhibition of full length wild type human N-terminal GST-tagged MALT1 catalytic domain (325 to 760 residues) expressed in Escherichia coli BL21 (DE3)...More data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Inhibition of recombinant human cathepsin B expressed in Escherichia coli BL21 (DE3) using zRR-AMC as substrate measured after 2 hrs by fluorescence ...More data for this Ligand-Target Pair
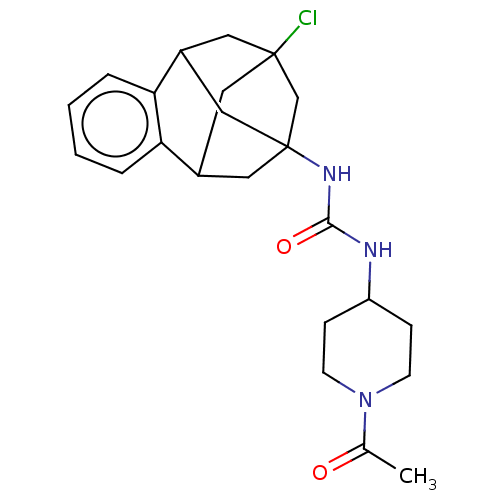
Affinity DataKi: 2.96E+3nMAssay Description:Inhibition of human neutrophil elastase using flurogenic substrate as elastase substrate V by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of recombinant human cathepsin B expressed in Escherichia coli BL21 (DE3) using zRR-AMC as substrate measured after 2 hrs by fluorescence ...More data for this Ligand-Target Pair
Affinity DataKi: 2.65E+4nMAssay Description:Inhibition of human proteinase 3 using flurogenic substrate as elastase substrate V by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.10E+4nMAssay Description:Inhibition of human neutrophil elastase using flurogenic substrate as elastase substrate V by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.38E+4nMAssay Description:Inhibition of human proteinase 3 using flurogenic substrate as elastase substrate V by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of FP-Rh binding to APT-1/2 in mouse brain cytosolic fraction preincubated for 1 hr followed by FP-Rh addition and measured after 1 hr by ...More data for this Ligand-Target Pair

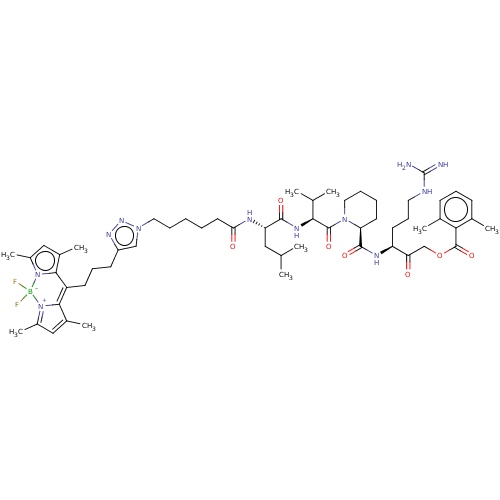




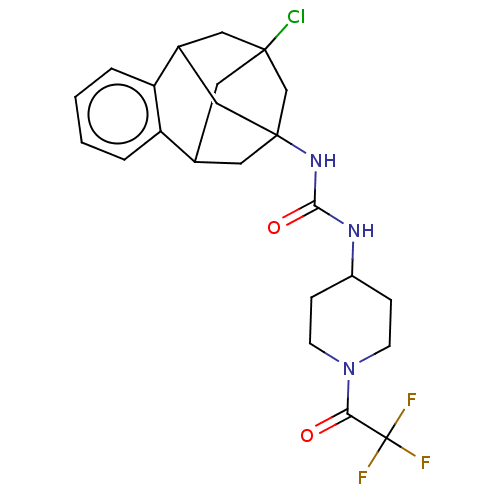

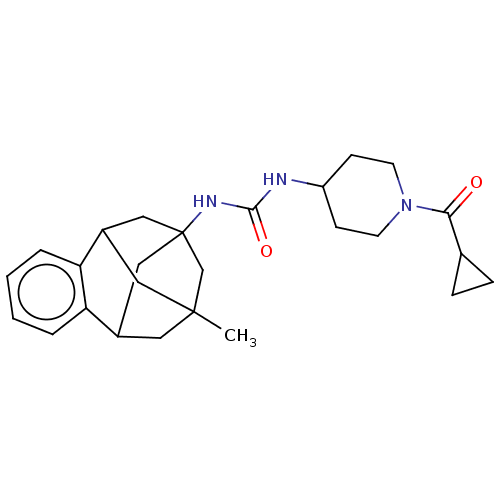
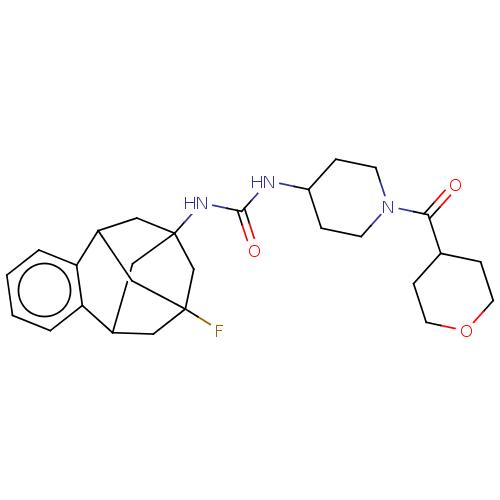
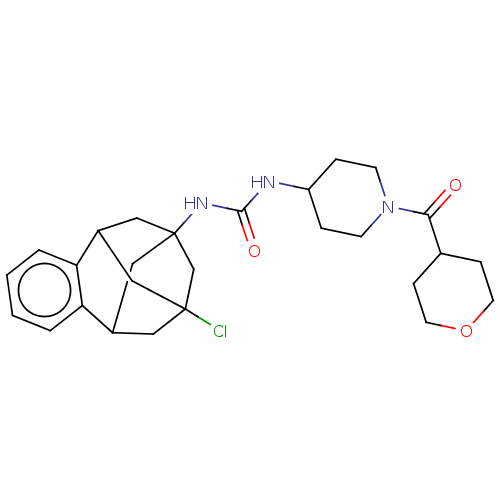


 3D Structure (crystal)
3D Structure (crystal)