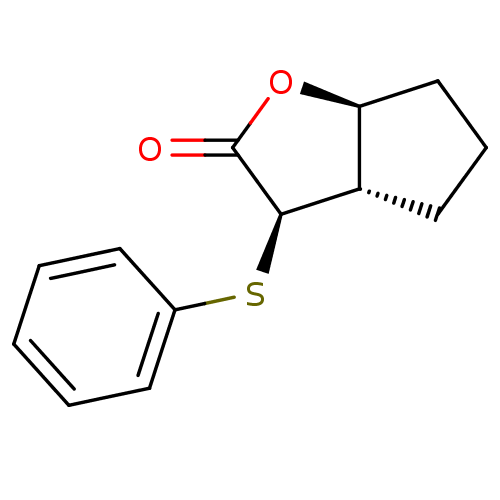
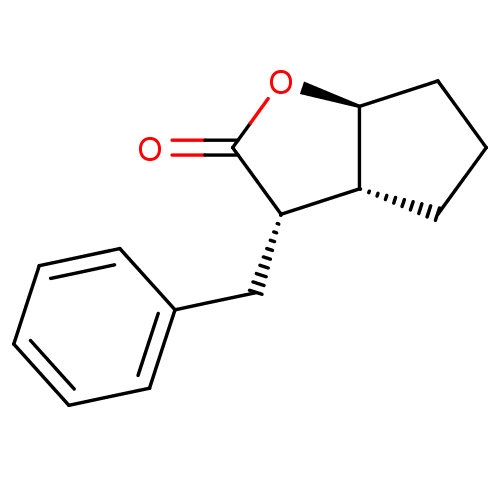
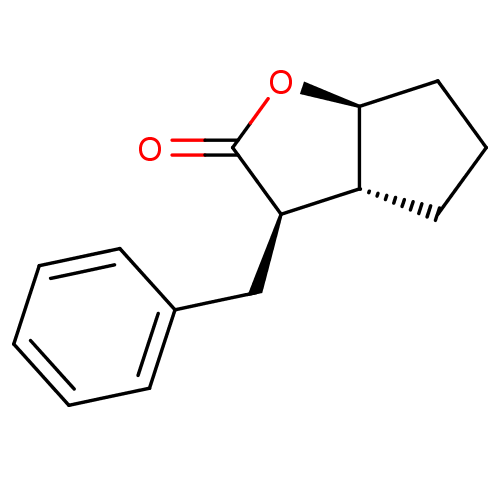
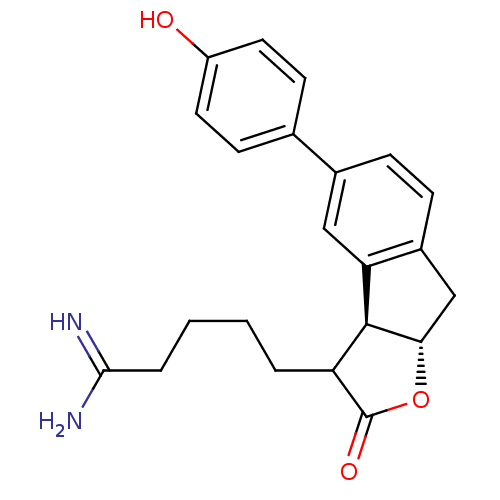
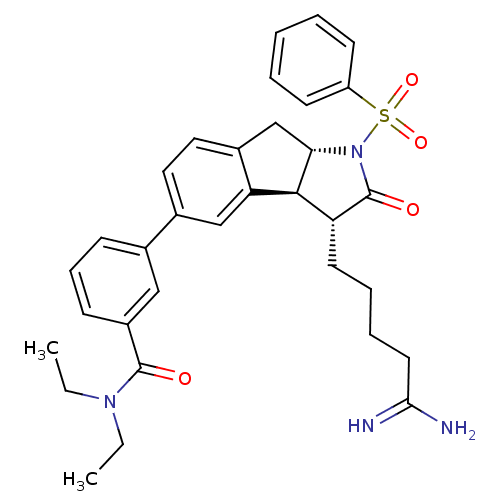
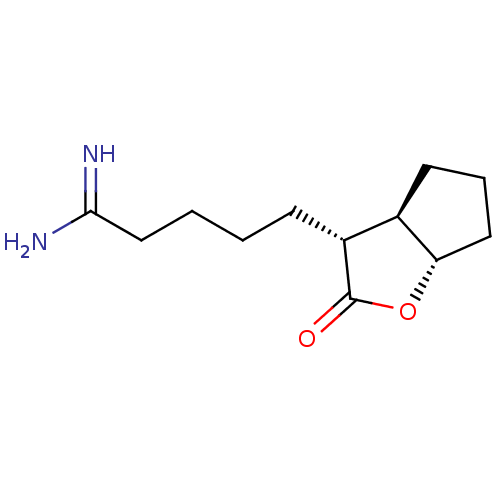
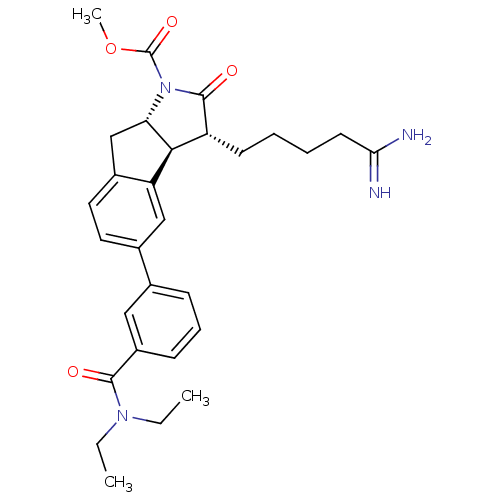
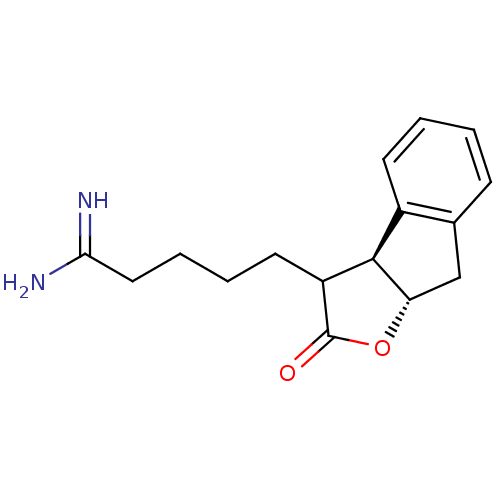
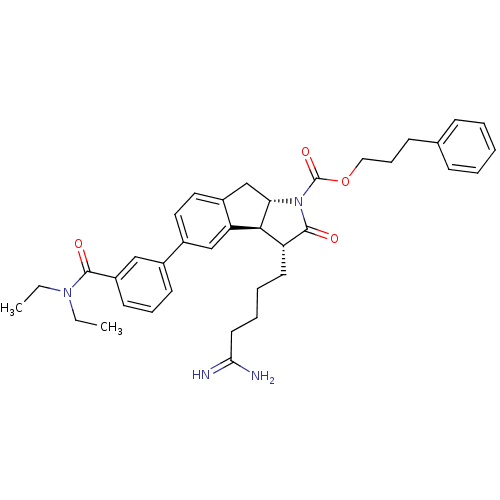
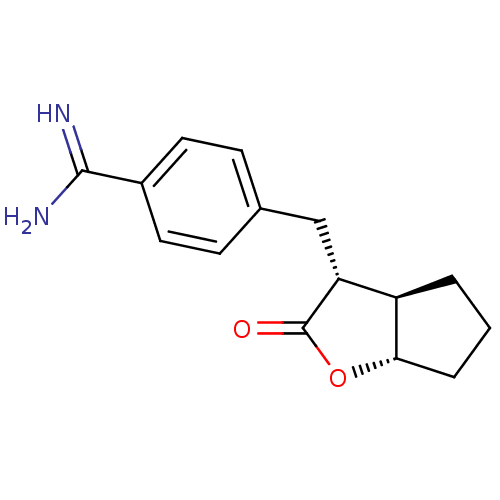
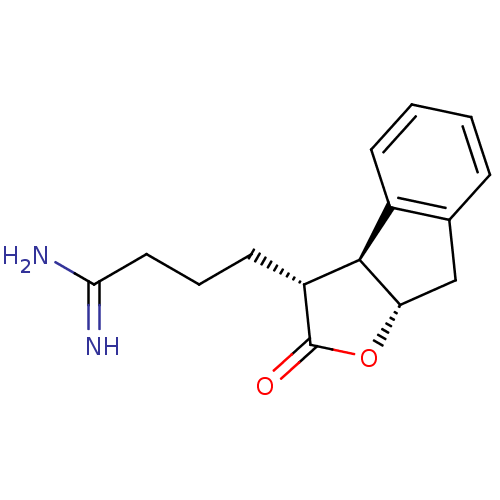
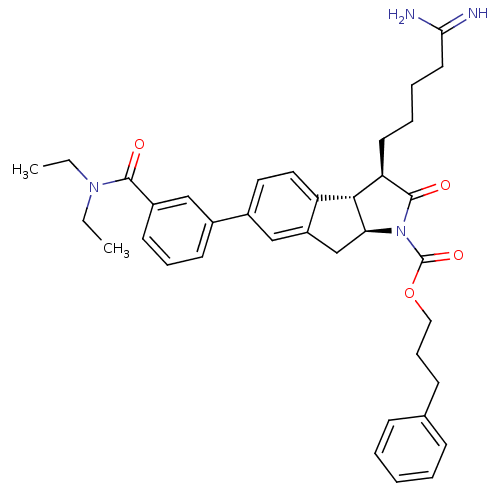
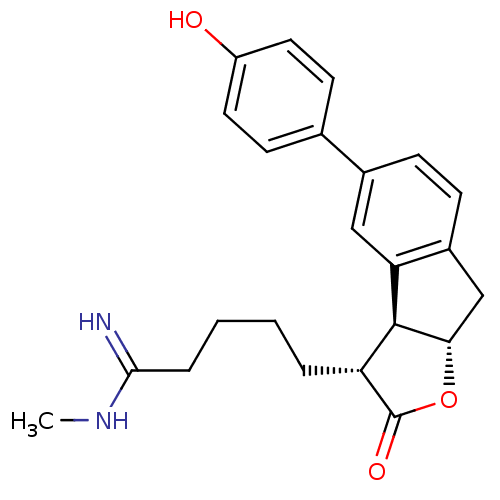
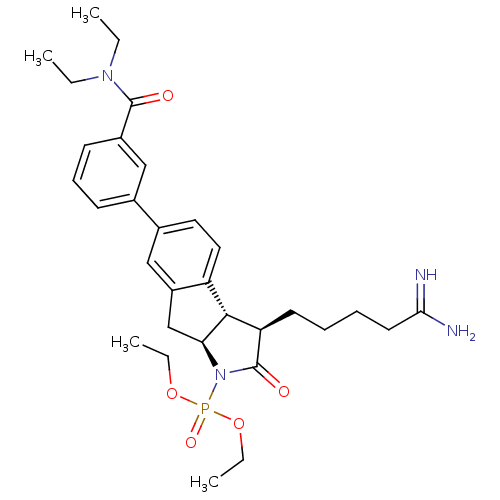
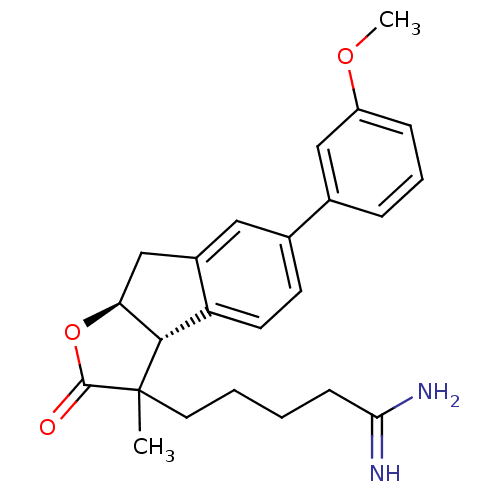
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.0200nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.120nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Compound was evaluated for the inhibition of Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataIC50: 73nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Glaxo Wellcome Research and Development
Curated by ChEMBL
Glaxo Wellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 74nMAssay Description:Inhibition of activated Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataIC50: 77nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 92nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 98nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Glaxo Wellcome Research and Development
Curated by ChEMBL
Glaxo Wellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of activated Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 620nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 660nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Glaxo Wellcome Research and Development
Curated by ChEMBL
Glaxo Wellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of activated Coagulation factor XMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair