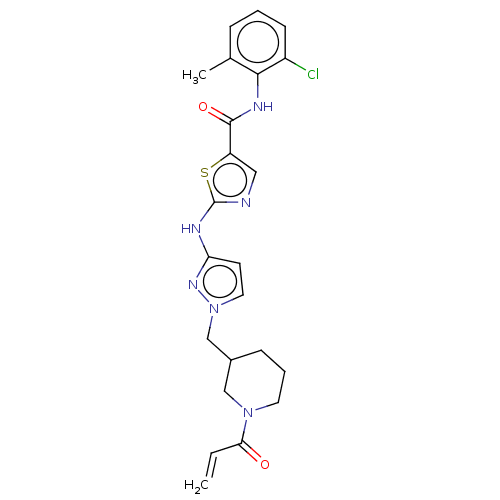
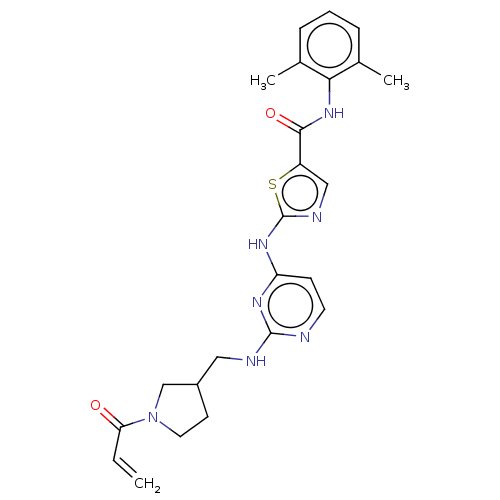
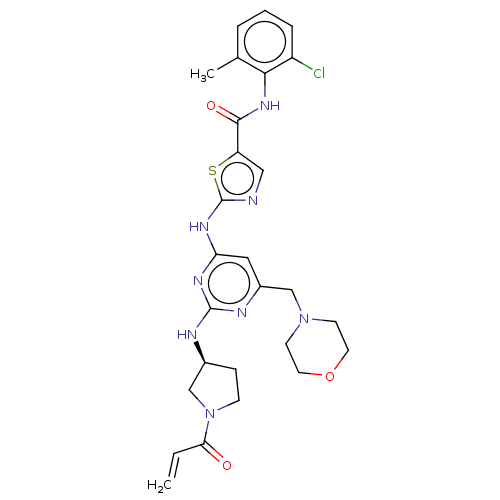
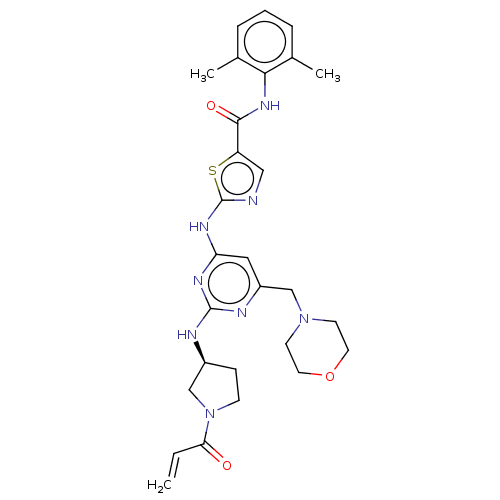
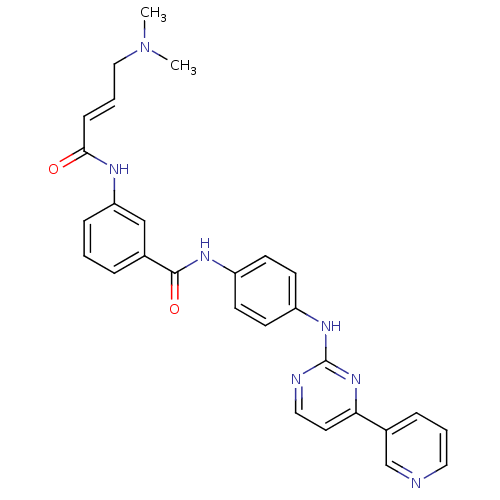
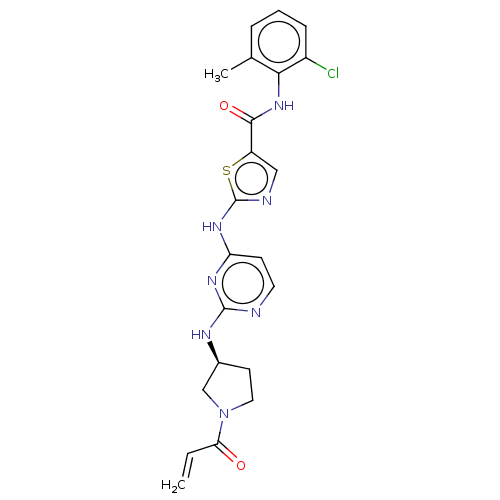
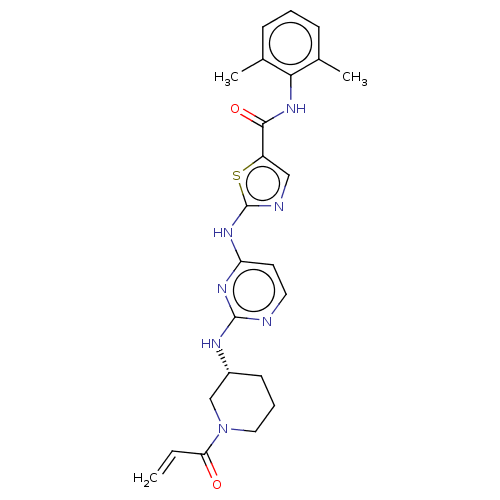
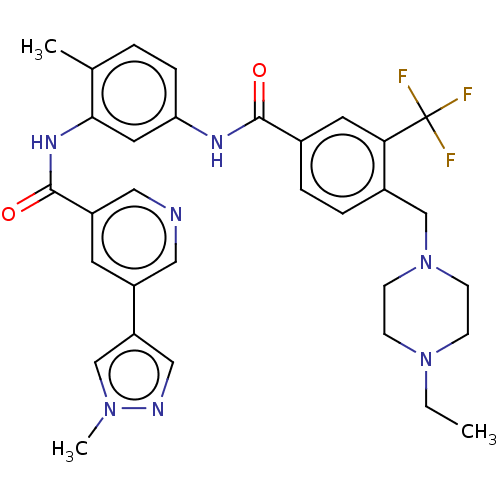
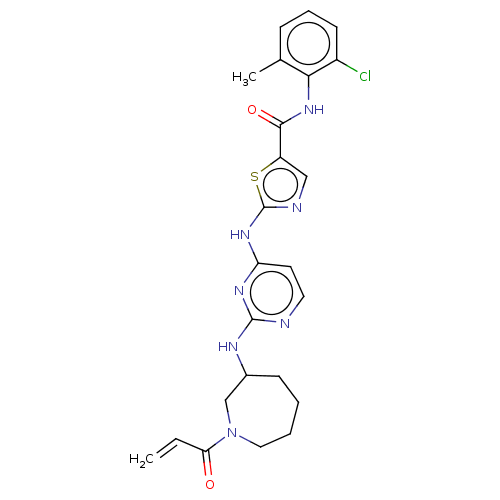
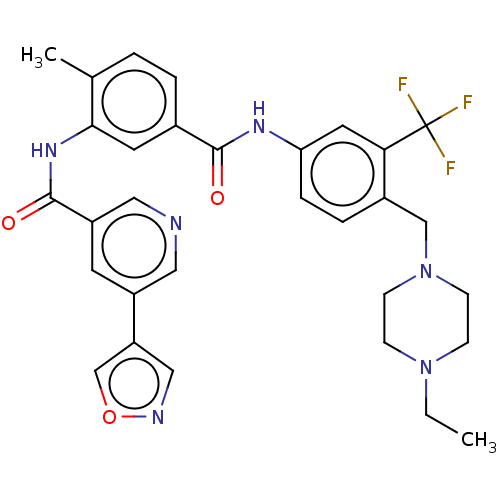
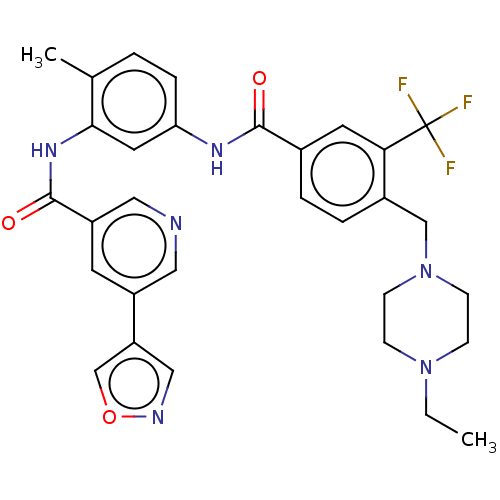
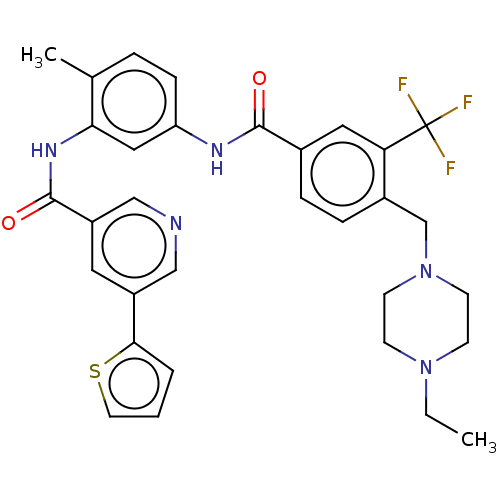
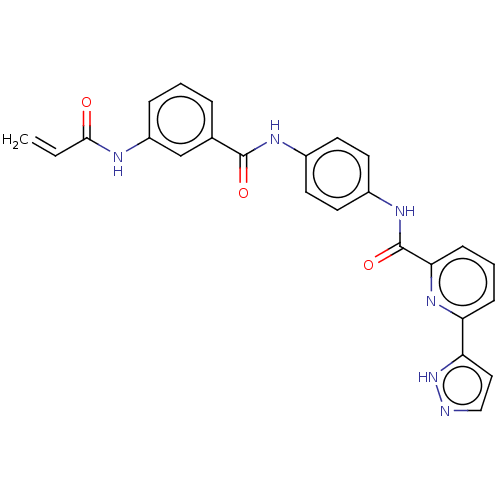
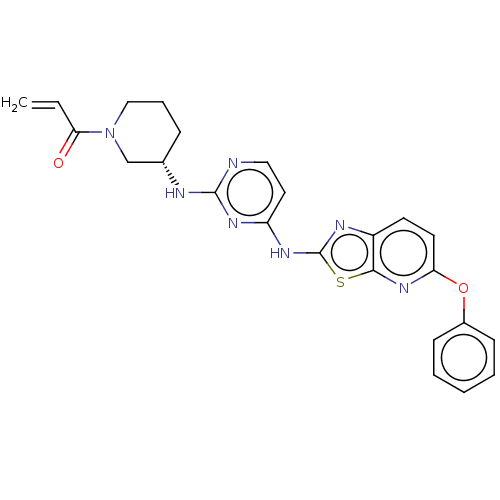
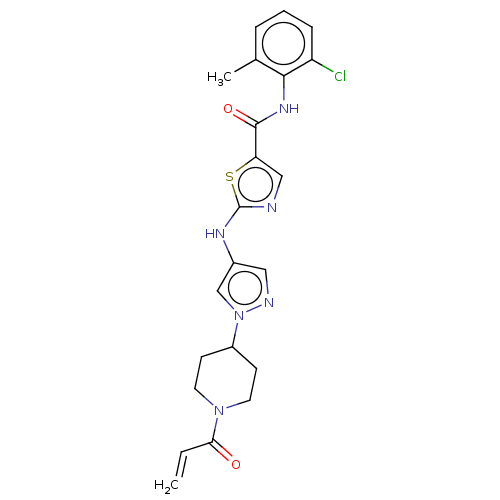
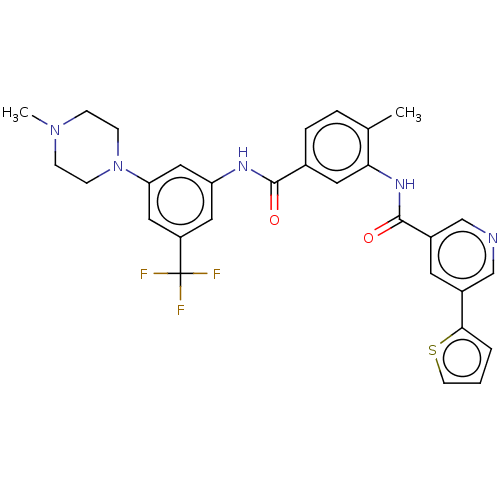
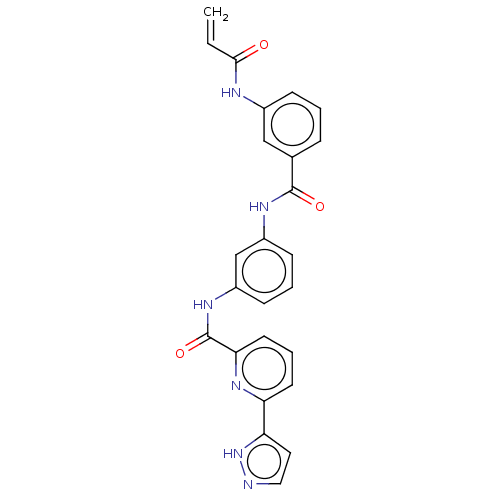
Affinity DataIC50: 0.495nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
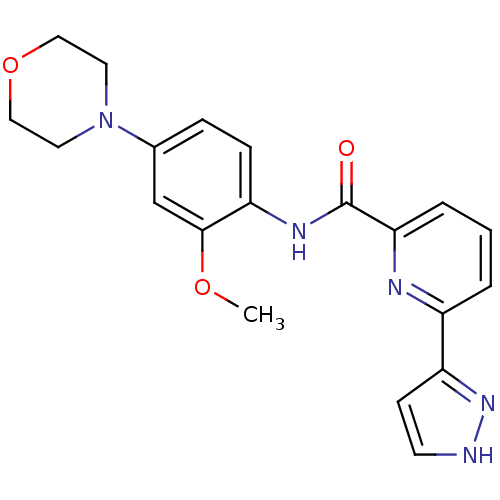
Affinity DataIC50: 0.495nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
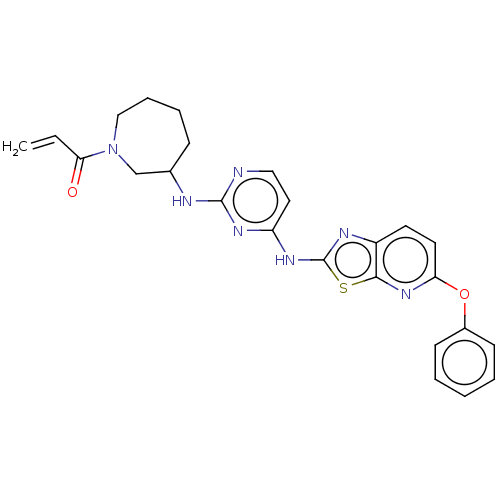
Affinity DataIC50: 0.495nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
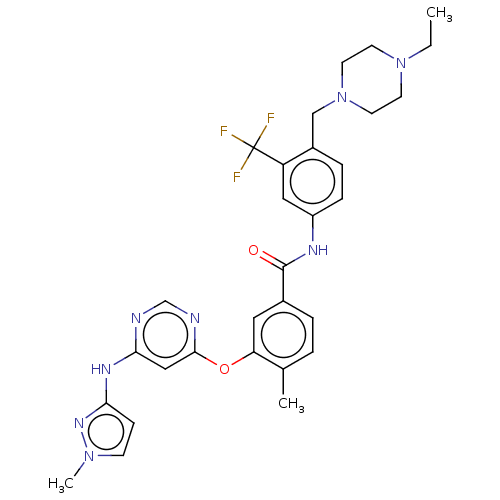
Affinity DataIC50: 0.495nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 0.495nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 0.495nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 0.495nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 0.730nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 0.75nMAssay Description:Inhibition of wild-type human partial length JNK3 (V28 to Q422 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 1.38nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of wild-type human full length JNK1 (M1 to Q384 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.61nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 1.63nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 1.64nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 1.74nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 1.94nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of wild-type human full length JNK2 (M1 to Q382 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.42nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 2.43nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 2.43nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
Affinity DataIC50: 3.99nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 4.24nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 5.33nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 5.33nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
Affinity DataIC50: 5.52nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 7.40nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
Affinity DataIC50: 7.40nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of recombinant IRAK1 (unknown origin) by Invitrogen adapta assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of wild-type human partial length IRAK1 (R194 to S712 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9.06nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of wild-type human partial length IRAK1 (R194 to S712 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of wild-type human partial length IRAK4 (M1 to S460 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
Affinity DataIC50: 19.4nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 19.9nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 22.8nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 22.8nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
Affinity DataIC50: 22.9nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
Affinity DataIC50: 22.9nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of recombinant IRAK1 (unknown origin) by Invitrogen adapta assayMore data for this Ligand-Target Pair
Affinity DataIC50: 25.3nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
Affinity DataIC50: 25.3nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
Affinity DataIC50: 25.3nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair