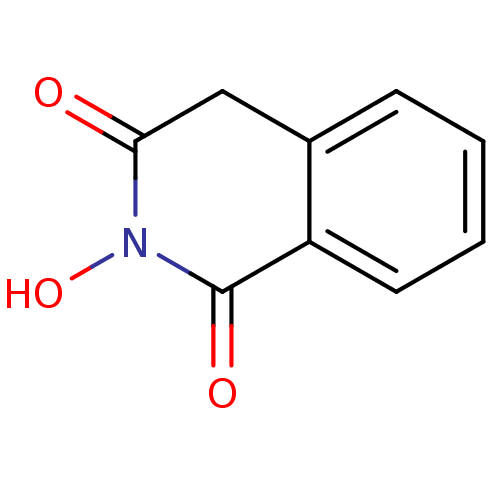
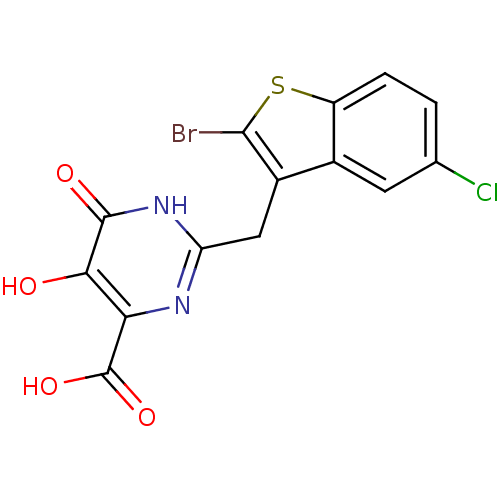
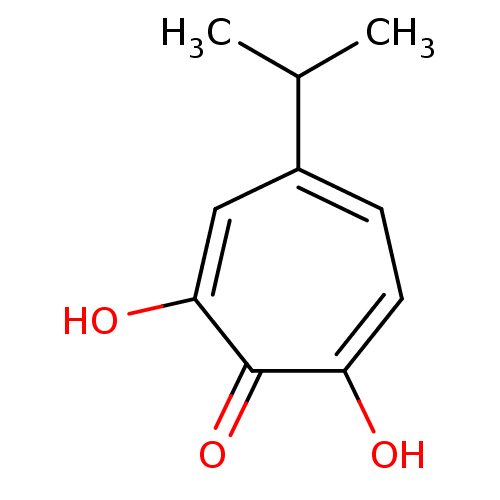
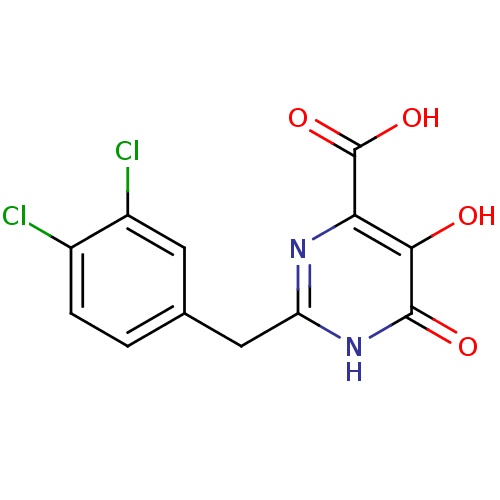
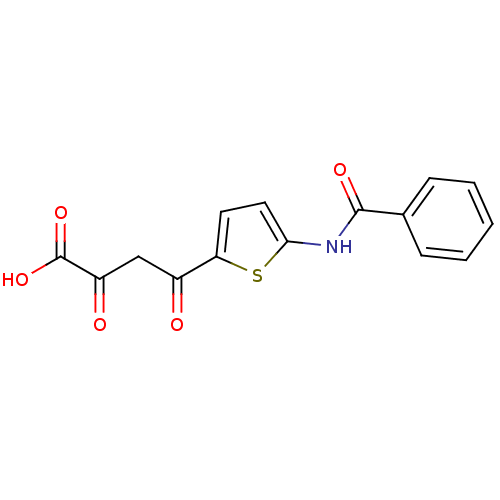
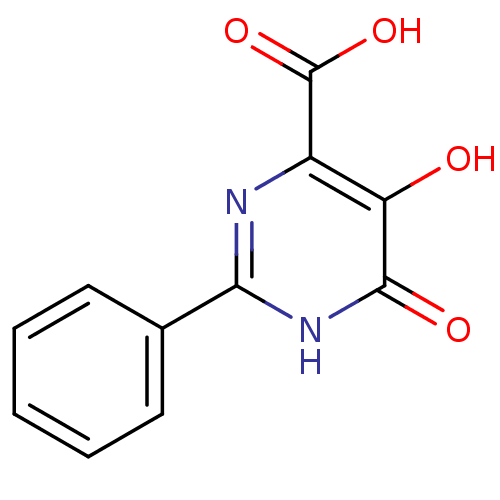
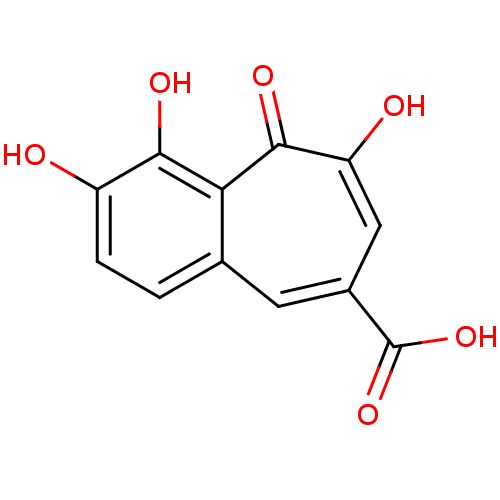
Affinity DataKi: 0.00500nMAssay Description:Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.00700nMAssay Description:Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0200nMAssay Description:Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0220nMAssay Description:Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0250nMAssay Description:Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0320nMAssay Description:Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0480nMAssay Description:Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0490nMAssay Description:Binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.129nMAssay Description:Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.129nMAssay Description:Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.240nMAssay Description:Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.417nMAssay Description:Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cellsMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
Section of Pharmacology
Curated by PDSP Ki Database
Section of Pharmacology
Curated by PDSP Ki Database
TargetMu-type opioid receptor(Homo sapiens (Human))
Section of Pharmacology
Curated by PDSP Ki Database
Section of Pharmacology
Curated by PDSP Ki Database
TargetDelta-type opioid receptor(Homo sapiens (Human))
Section of Pharmacology
Curated by PDSP Ki Database
Section of Pharmacology
Curated by PDSP Ki Database
Affinity DataIC50: 19nMAssay Description:Inhibition of HSP90 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 135nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 175nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 660nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.15E+3nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.45E+3nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 9.85E+3nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 2.32E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 4.85E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataEC50: 5.40nMAssay Description:Inhibition of electrically evoked contraction of mouse vas deferensMore data for this Ligand-Target Pair
Affinity DataEC50: 4.90nMAssay Description:Inhibition of forskolin stimulated cAMP levels in CHO cell membranes expressing the human NOP receptor (CHOhNOP)More data for this Ligand-Target Pair




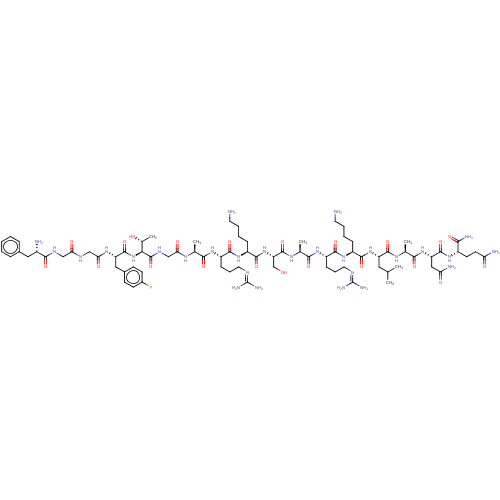
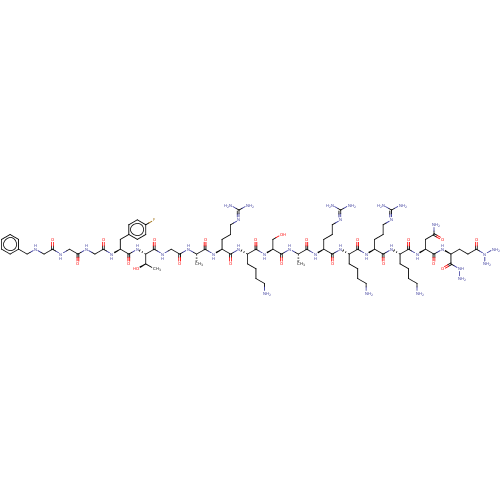
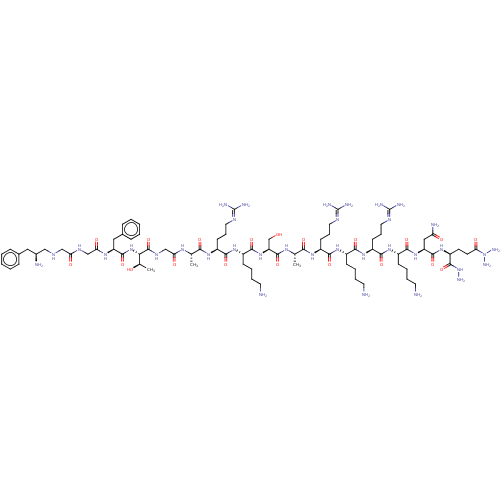
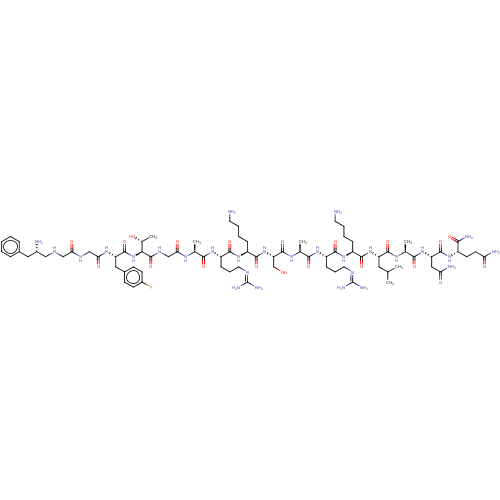
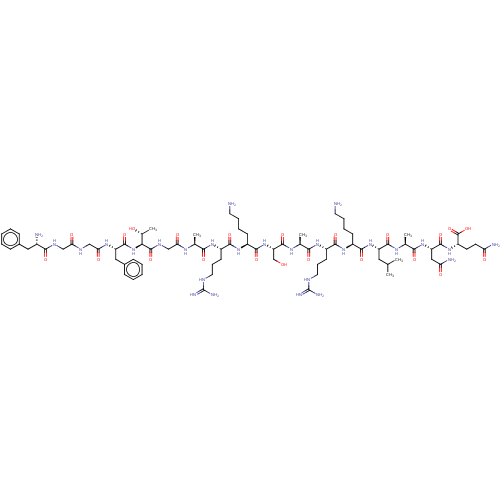
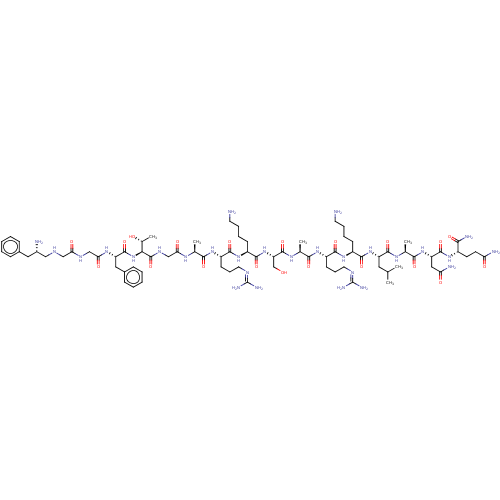

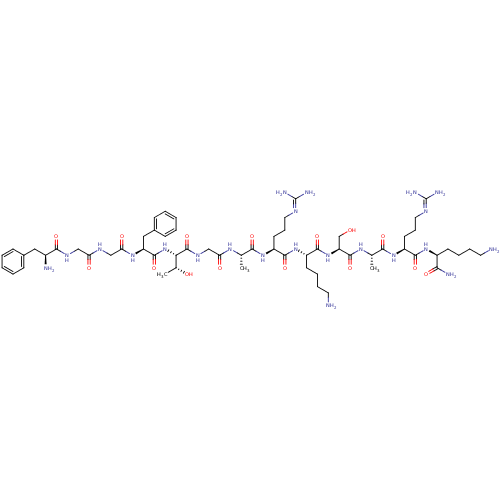
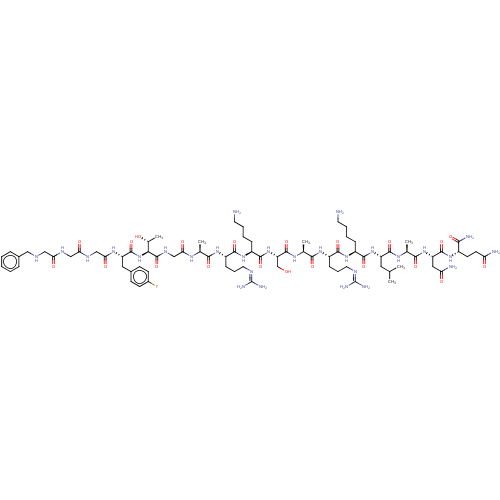
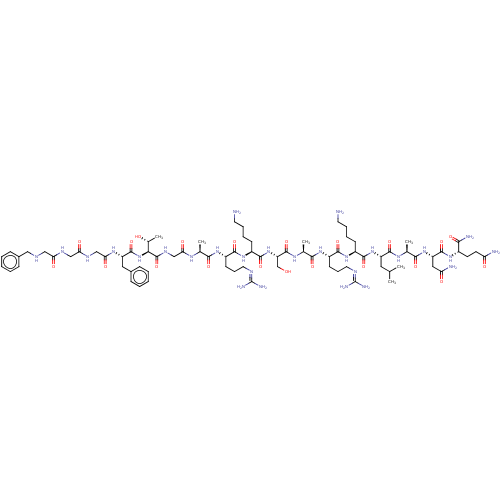
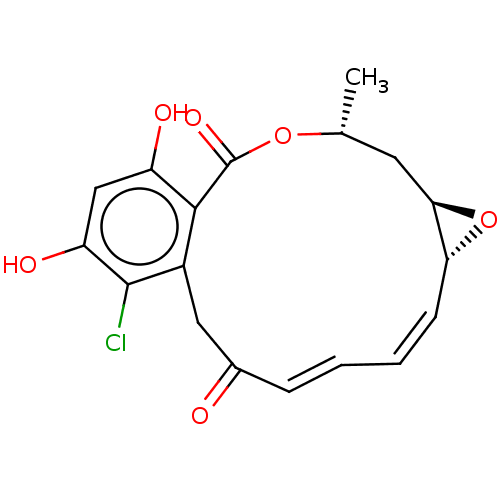
 3D Structure (crystal)
3D Structure (crystal)