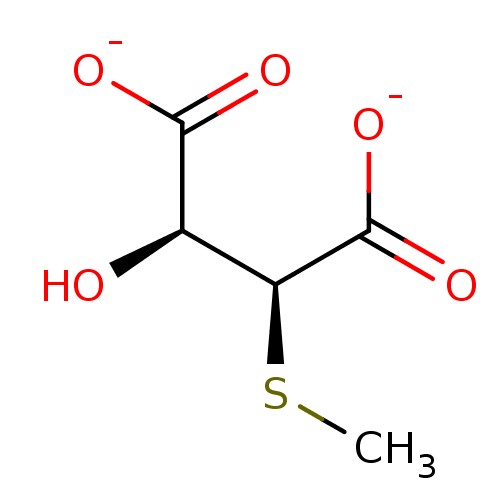
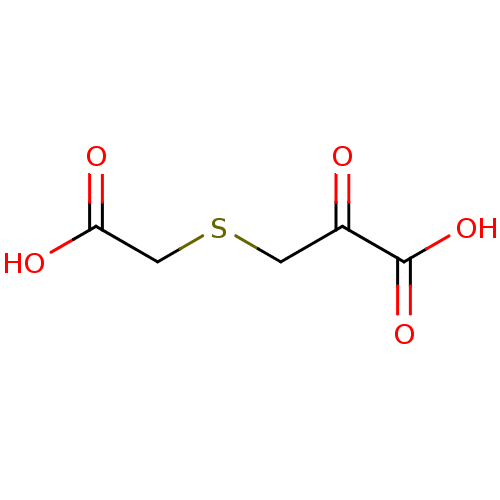
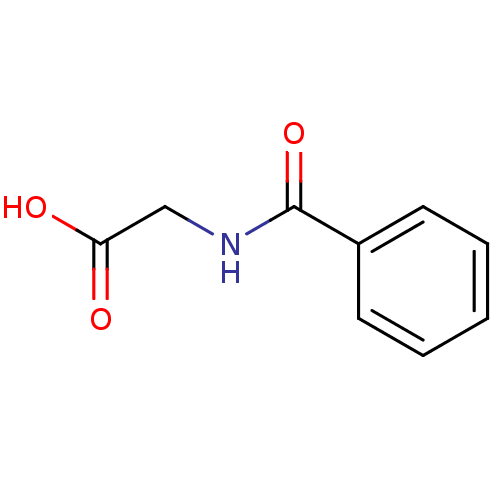
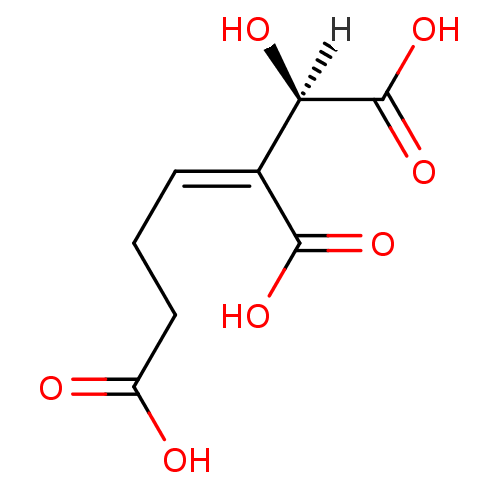
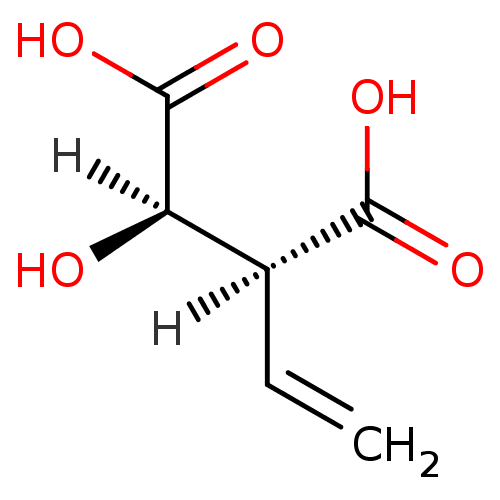
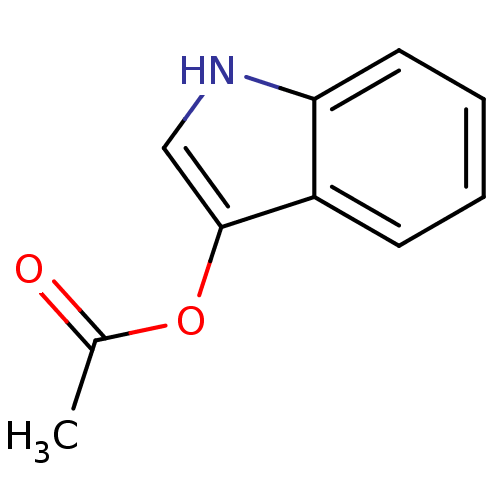
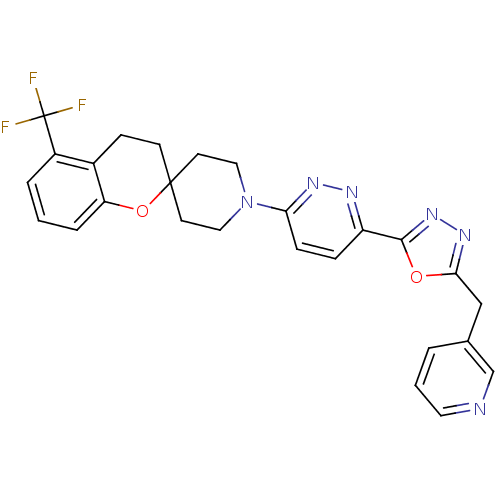
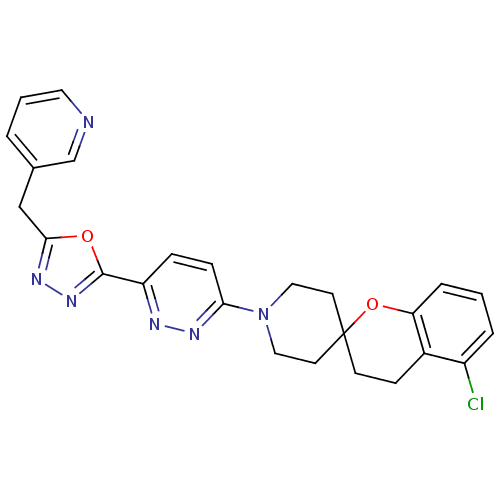
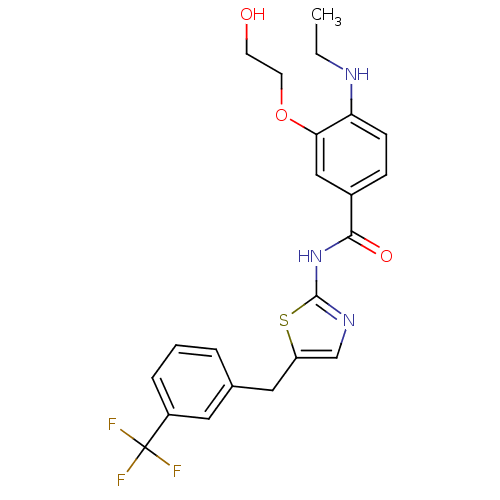
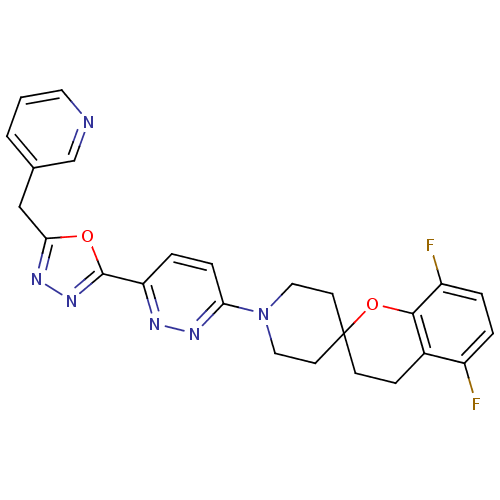
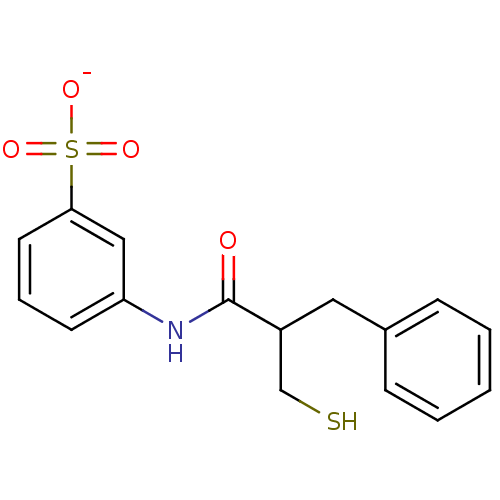
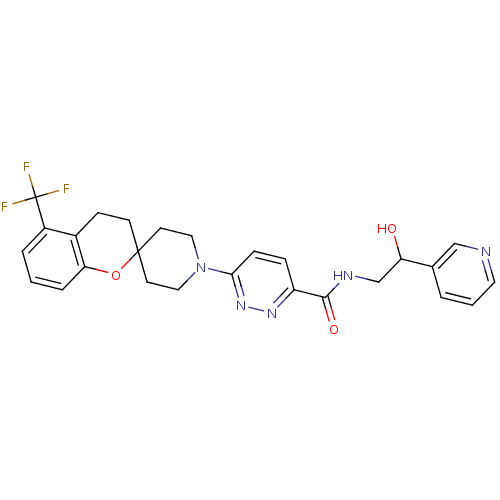
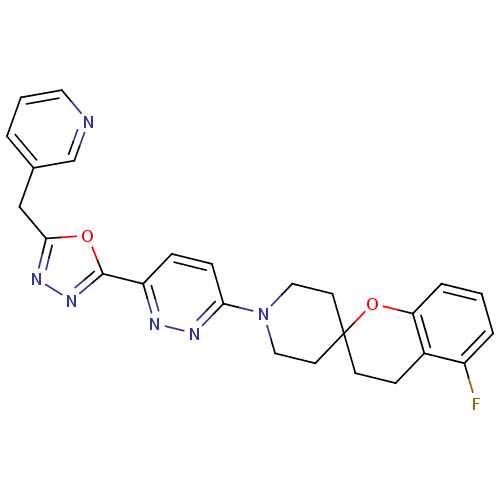
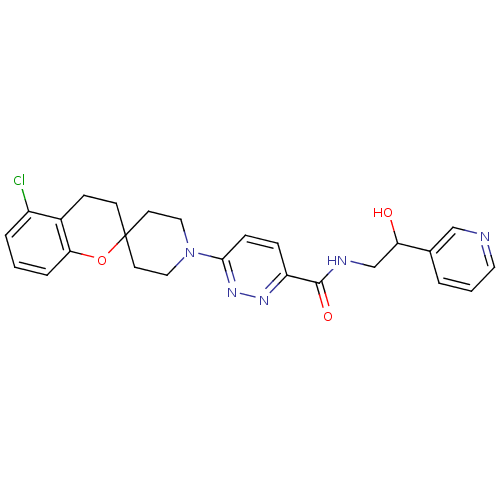
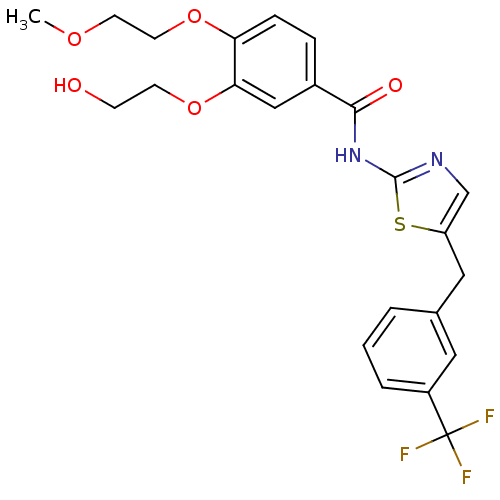
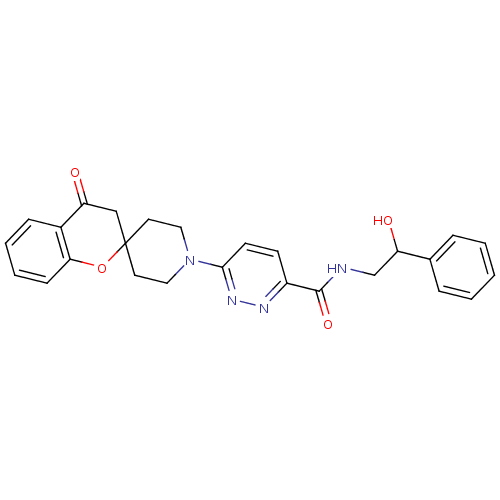
Target3-isopropylmalate dehydrogenase(Thermus thermophilus)
Tokyo Institute of Technology
Curated by ChEMBL
Tokyo Institute of Technology
Curated by ChEMBL
Affinity DataKi: 62nMAssay Description:Inhibition of Thermus thermophilus HB8 IPMDH assessed as formation of NADH by Lineweaver-Burke plot analysisMore data for this Ligand-Target Pair
TargetHomoisocitrate dehydrogenase, mitochondrial(Saccharomyces cerevisiae)
Tokyo Institute of Technology
Tokyo Institute of Technology
Affinity DataKi: 97nM ΔG°: -41.5kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
TargetHomoisocitrate dehydrogenase, mitochondrial(Saccharomyces cerevisiae)
Tokyo Institute of Technology
Tokyo Institute of Technology
Affinity DataKi: 1.00E+4nM ΔG°: -29.6kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
TargetHomoisocitrate dehydrogenase, mitochondrial(Saccharomyces cerevisiae)
Tokyo Institute of Technology
Tokyo Institute of Technology
Affinity DataKi: 1.20E+4nM ΔG°: -29.1kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+4nMAssay Description:TP_TRANSPORTER: inhibition of benzylpenicillin uptake in Oat3-expressing HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.10E+4nMAssay Description:TP_TRANSPORTER: inhibition of benzylpenicillin uptake in OAT3-expressing HEK293 cellsMore data for this Ligand-Target Pair
TargetHomoisocitrate dehydrogenase, mitochondrial(Saccharomyces cerevisiae)
Tokyo Institute of Technology
Tokyo Institute of Technology
Affinity DataKi: 7.20E+4nM ΔG°: -24.5kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
Affinity DataKi: 8.80E+4nM ΔG°: -23.4kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
Affinity DataKi: 2.60E+5nM ΔG°: -20.7kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
Affinity DataKi: 4.91E+5nMAssay Description:TP_TRANSPORTER: inhibition of benzylpenicillin uptake in OAT3-expressing HEK293 cellsMore data for this Ligand-Target Pair
TargetHomoisocitrate dehydrogenase, mitochondrial(Saccharomyces cerevisiae)
Tokyo Institute of Technology
Tokyo Institute of Technology
Affinity DataKi: 5.10E+5nM ΔG°: -19.5kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
Affinity DataKi: 5.82E+5nMAssay Description:TP_TRANSPORTER: inhibition of benzylpenicillin uptake in Oat3-expressing HEK293 cellsMore data for this Ligand-Target Pair
TargetHomoisocitrate dehydrogenase, mitochondrial(Saccharomyces cerevisiae)
Tokyo Institute of Technology
Tokyo Institute of Technology
Affinity DataKi: 7.90E+5nM ΔG°: -18.4kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
TargetHomoisocitrate dehydrogenase, mitochondrial(Saccharomyces cerevisiae)
Tokyo Institute of Technology
Tokyo Institute of Technology
Affinity DataKi: 1.40E+6nM ΔG°: -16.9kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
Affinity DataKi: 3.10E+6nM ΔG°: -14.5kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
Affinity DataKi: 5.20E+6nM ΔG°: -13.2kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
Affinity DataKi: 1.53E+7nM ΔG°: -10.5kJ/molepH: 7.8 T: 2°CAssay Description:Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of human SCD1More data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of SCD1 in mouse microsomal liver S9 microsomal fraction assessed as conversion of [14C]stearate to [14C]oleate after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0400nMAssay Description:Inhibition of SCD1 in mouse microsome assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0400nMAssay Description:Inhibition of stearoyl-CoA desaturase 1 in human microsome assessed as conversion of [14C]stearate to [14C]oleateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0600nMAssay Description:Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0600nMAssay Description:Inhibition of SCD1 in mouse microsome assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0600nMAssay Description:Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fractionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0680nMAssay Description:Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibition of SCD1 in mouse microsomal liver S9 microsomal fraction assessed as conversion of [14C]stearate to [14C]oleate after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of SCD1 in mouse microsome assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.270nMAssay Description:In vitro inhibitory activity against enkephalinase enzyme from rat.More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:Inhibition of SCD1 in mouse microsomal liver S9 microsomal fraction assessed as conversion of [14C]stearate to [14C]oleate after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of stearoyl-CoA desaturase 1 in human microsome assessed as conversion of [14C]stearate to [14C]oleateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of stearoyl-CoA desaturase 1 in mouse microsome assessed as conversion of [14C]stearate to [14C]oleateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.450nMAssay Description:Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fractionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of SCD1 in mouse microsome assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.560nMAssay Description:Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of SCD1 in mouse microsomal liver S9 microsomal fraction assessed as conversion of [14C]stearate to [14C]oleate after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of stearoyl-CoA desaturase 1 in mouse microsome assessed as conversion of [14C]stearate to [14C]oleateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of stearoyl-CoA desaturase 1 in human microsome assessed as conversion of [14C]stearate to [14C]oleateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.690nMAssay Description:Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of human SCD1 expressed in HEK293A cells assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 minsMore data for this Ligand-Target Pair