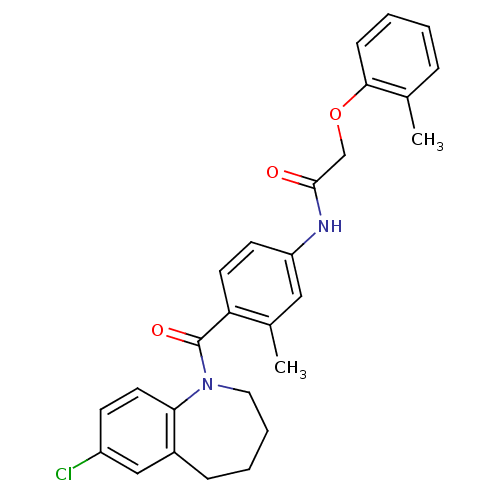
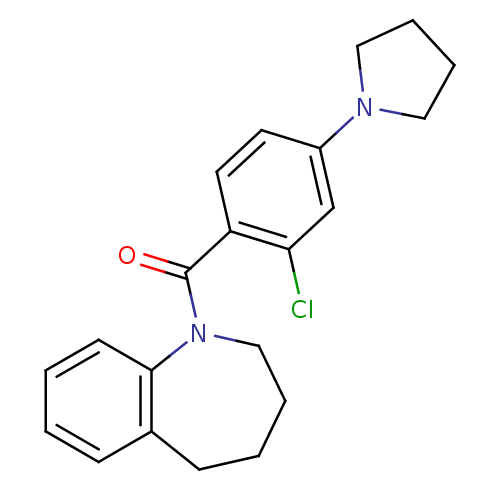
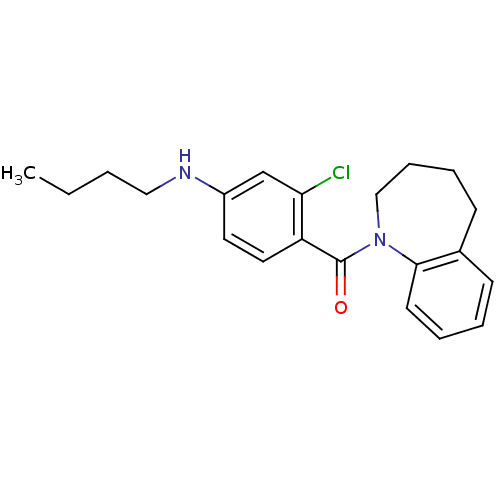
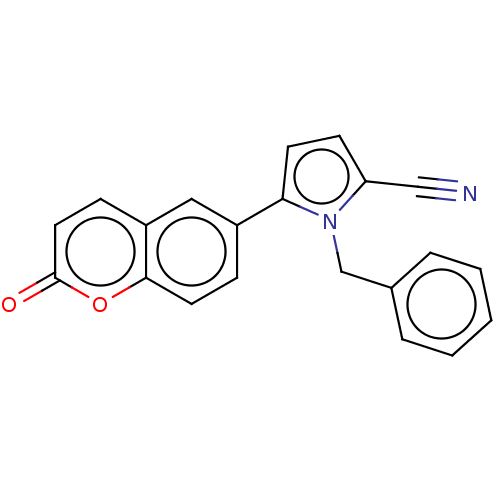
Affinity DataKi: 0.430nMAssay Description:Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50%More data for this Ligand-Target Pair
TargetVasopressin V1b receptor(Homo sapiens (Human))
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
TargetVasopressin V2 receptor(Rattus norvegicus (Rat))
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
TargetVasopressin V2 receptor(Rattus norvegicus (Rat))
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
TargetVasopressin V1a receptor(RAT)
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Tokyo Medical and Dental University (TMDU)
Curated by ChEMBL
Tokyo Medical and Dental University (TMDU)
Curated by ChEMBL
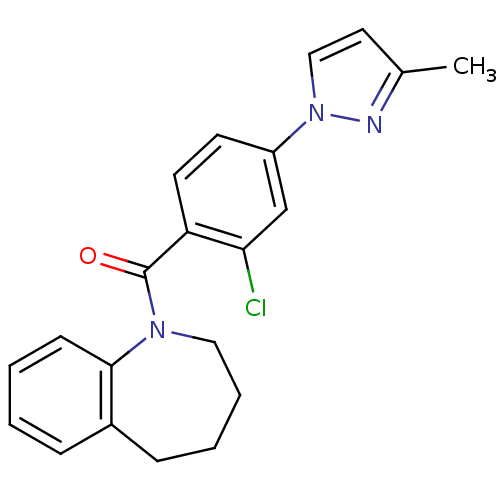
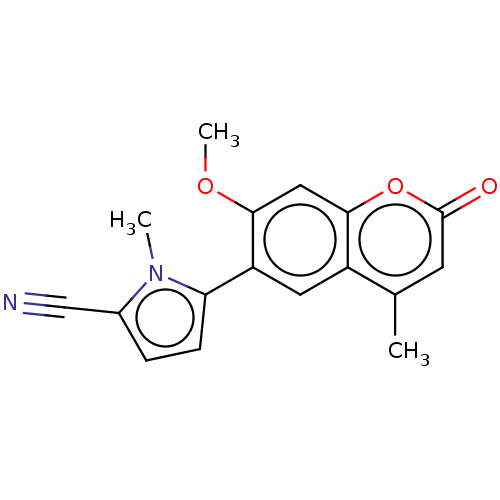
Affinity DataKi: 1.60nMAssay Description:Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysisMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Tokyo Medical and Dental University (TMDU)
Curated by ChEMBL
Tokyo Medical and Dental University (TMDU)
Curated by ChEMBL
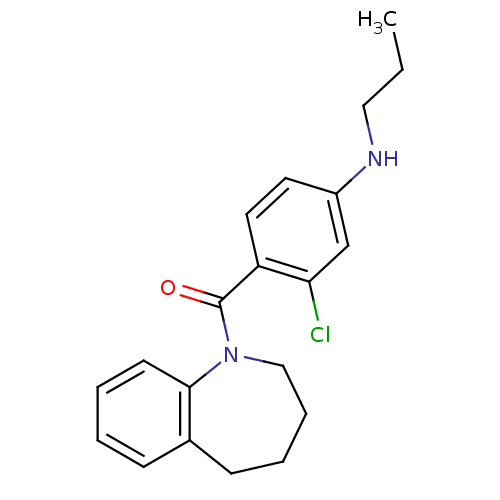
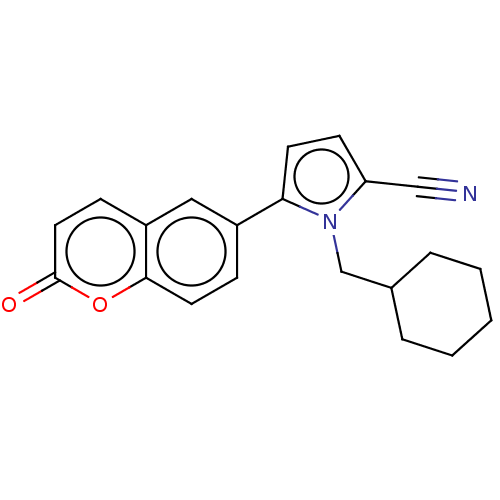
Affinity DataKi: 2.5nMAssay Description:Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysisMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Rattus norvegicus (Rat))
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Tokyo Medical and Dental University (TMDU)
Curated by ChEMBL
Tokyo Medical and Dental University (TMDU)
Curated by ChEMBL
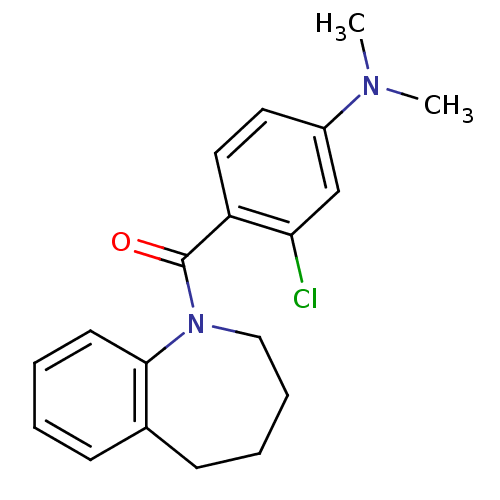
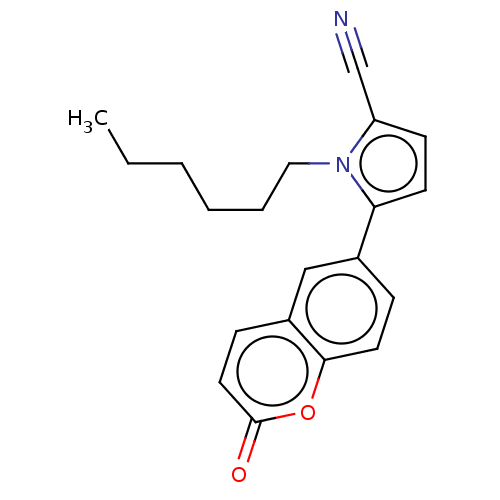
Affinity DataKi: 9nMAssay Description:Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysisMore data for this Ligand-Target Pair
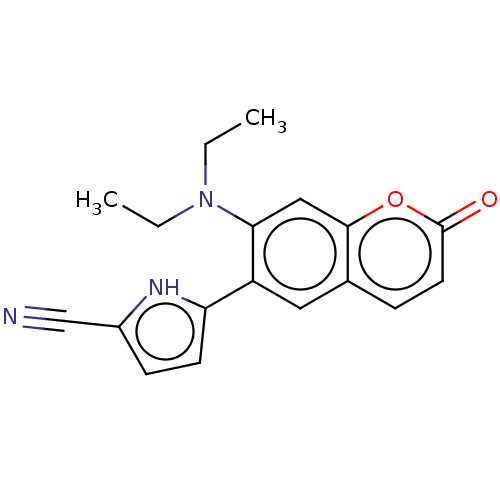
Affinity DataKi: 9.42nMAssay Description:Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50%More data for this Ligand-Target Pair
TargetVasopressin V1a receptor(Homo sapiens (Human))
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
TargetVasopressin V1a receptor(RAT)
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Affinity DataKi: 32nMAssay Description:Binding affinity of the compound towards Vasopressin V1a receptor in rat liverMore data for this Ligand-Target Pair
TargetVasopressin V1a receptor(Homo sapiens (Human))
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Tokyo Medical and Dental University (TMDU)
Curated by ChEMBL
Tokyo Medical and Dental University (TMDU)
Curated by ChEMBL
Affinity DataKi: 199nMAssay Description:Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysisMore data for this Ligand-Target Pair
TargetVasopressin V1a receptor(RAT)
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
TargetVasopressin V1a receptor(RAT)
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
TargetVasopressin V1b receptor(Homo sapiens (Human))
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
TargetVasopressin V1b receptor(Homo sapiens (Human))
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
TargetVasopressin V1a receptor(Homo sapiens (Human))
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Affinity DataKi: 1.40E+4nMAssay Description:Binding affinity of the compound towards Vasopressin V1a receptor in human liverMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Inhibition of recombinant Set7/9 (unknown origin) expressed in Escherichia coli BL21 (DE3) using Ac-KRSK-MCA peptide/SAM as substrate preincubated fo...More data for this Ligand-Target Pair
Affinity DataIC50: 0.950nMAssay Description:Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50%More data for this Ligand-Target Pair
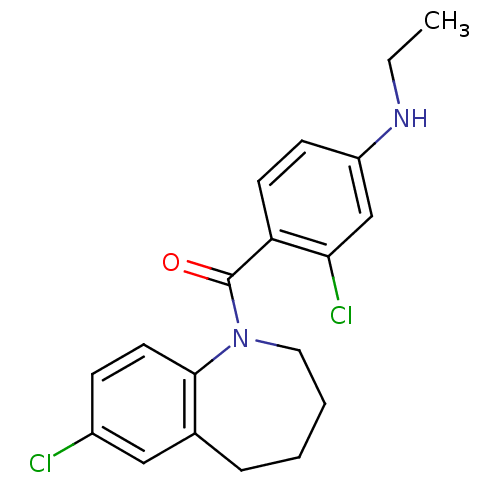
Affinity DataIC50: 4.10nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.90nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
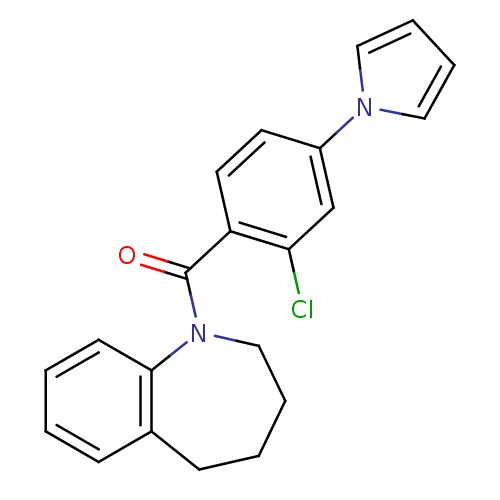
Affinity DataIC50: 7.5nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 8.80nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
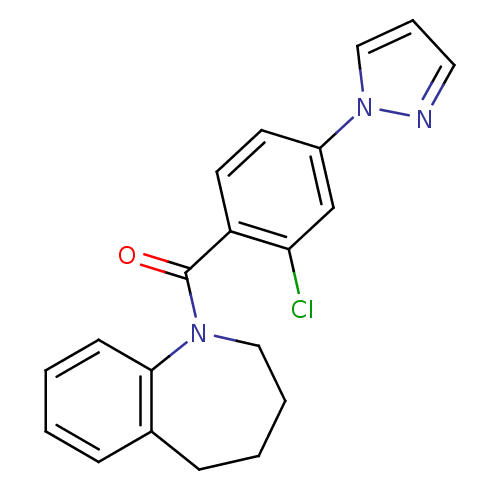
Affinity DataIC50: 9nMAssay Description:Binding affinity of the compound against human Vasopressin V2 receptor expressed in HeLa cells was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline...More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline...More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Rattus norvegicus (Rat))
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
Affinity DataIC50: 38nMAssay Description:Binding affinity of the compound towards rat Vasopressin V2 receptor after peroral administration of the compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline...More data for this Ligand-Target Pair
Affinity DataIC50: 65nMAssay Description:Antagonist activity at human progesterone receptor in T47D cells after 24 hrs by alkaline phosphatase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline...More data for this Ligand-Target Pair






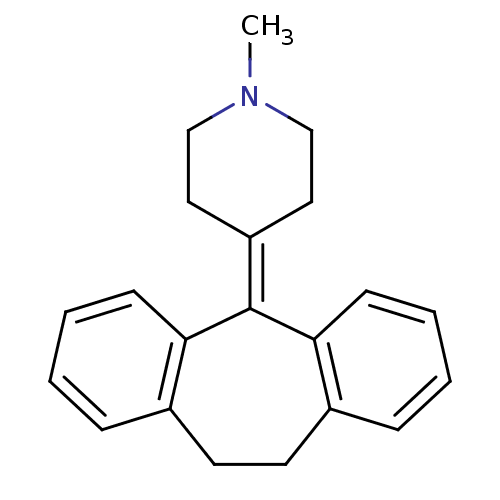


 3D Structure (crystal)
3D Structure (crystal)