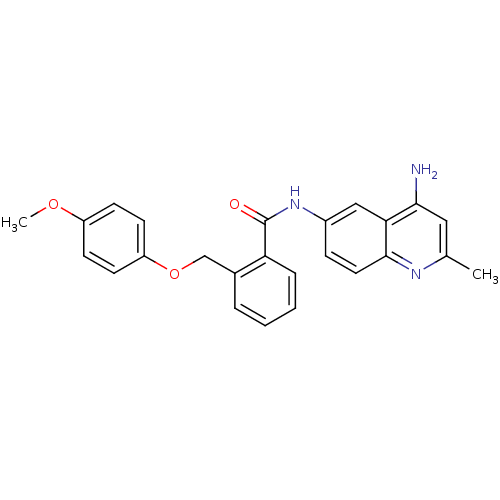
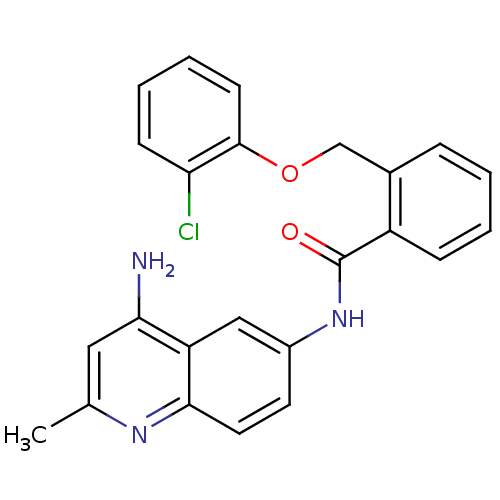
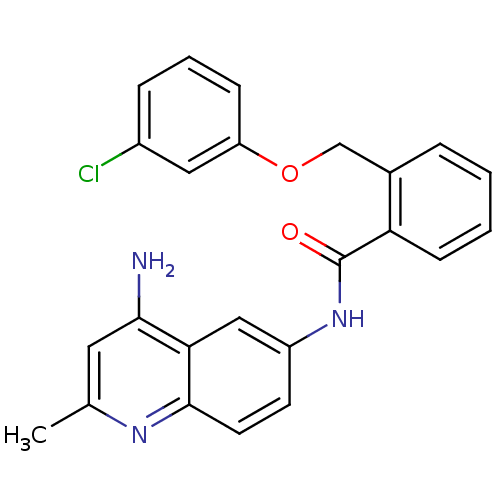
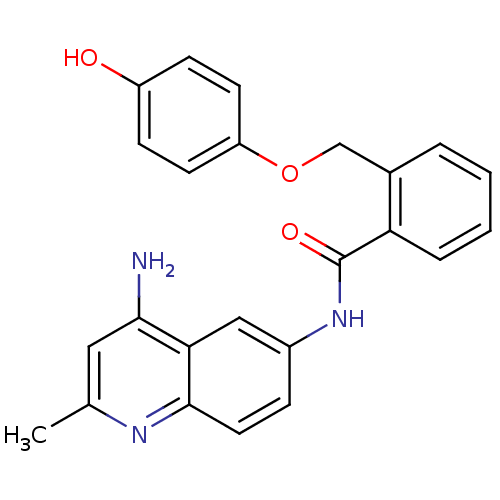
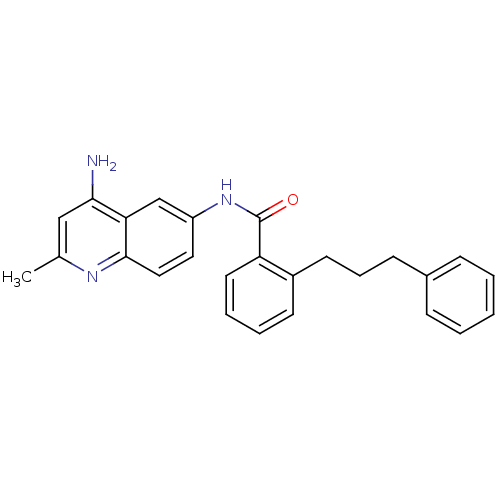
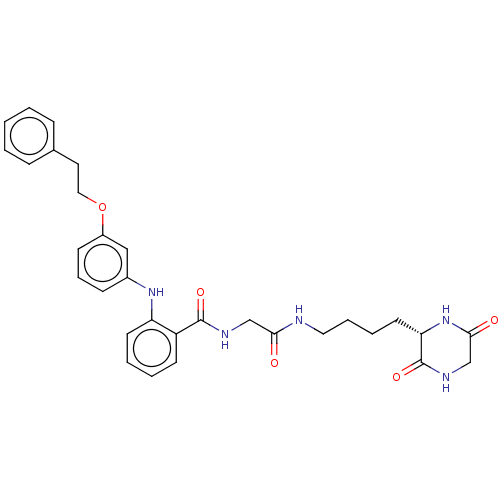
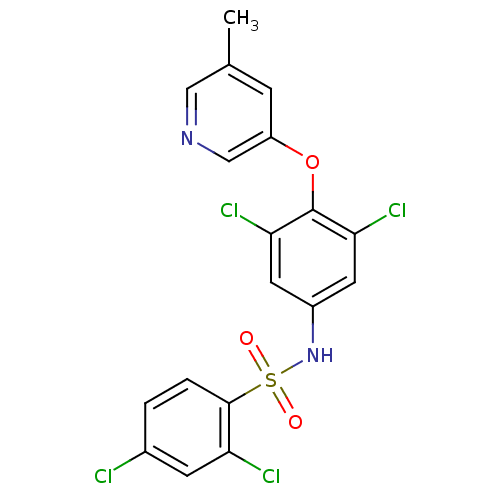
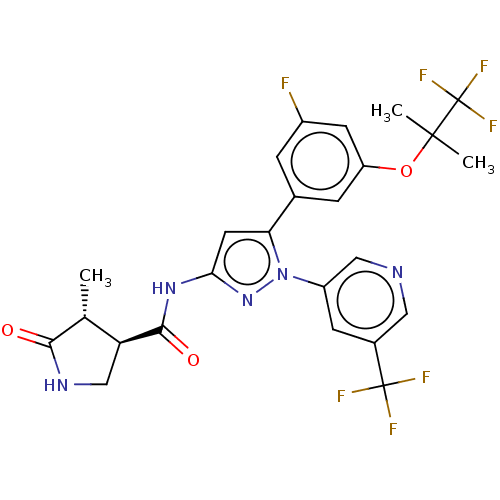
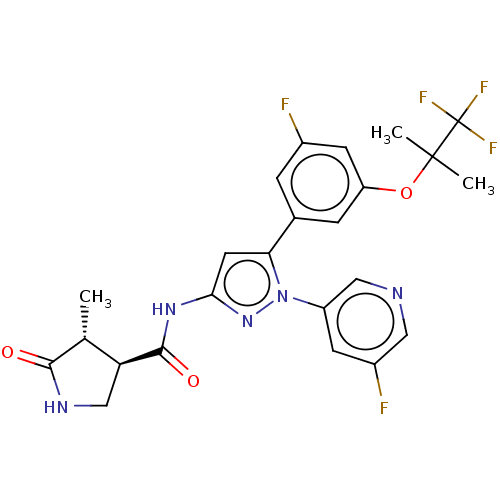
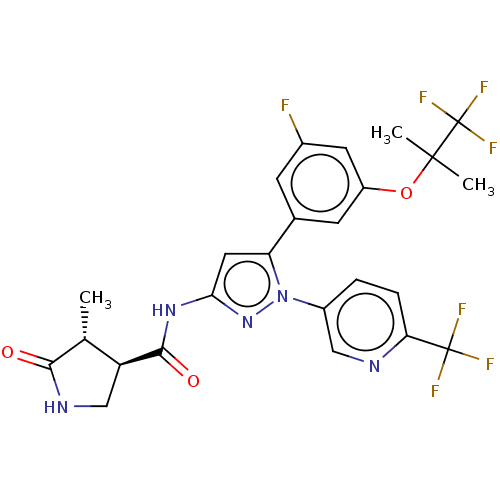
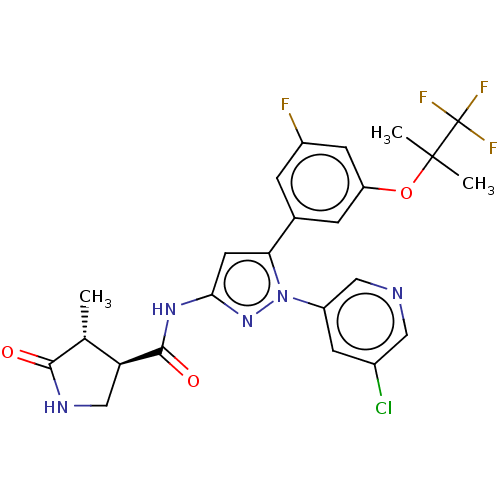
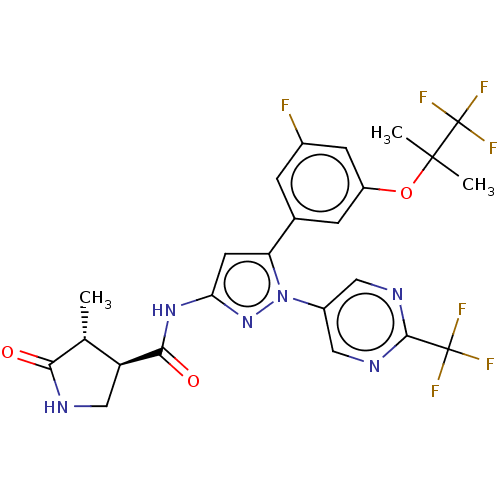
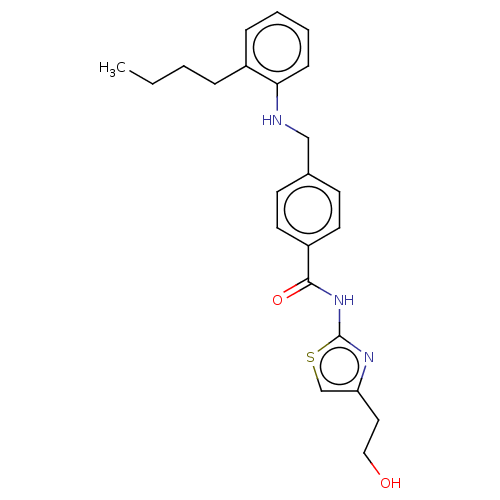
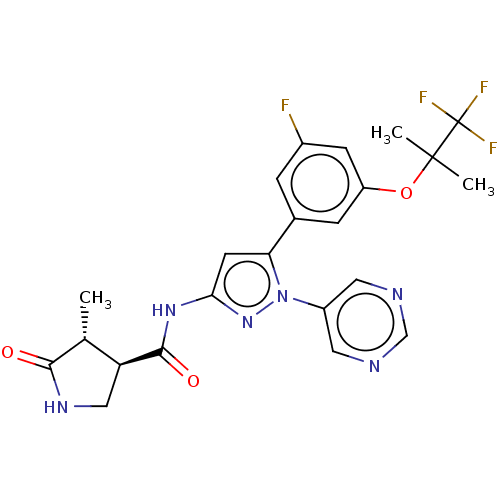
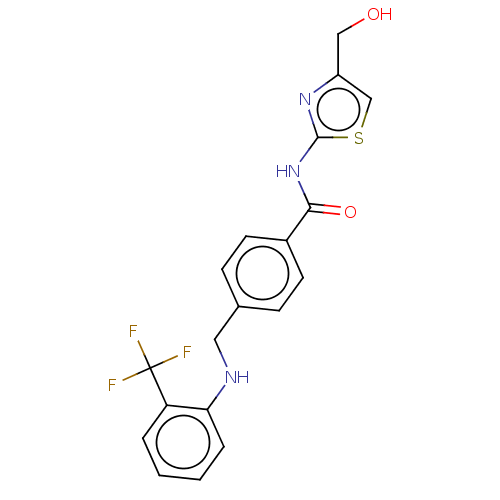
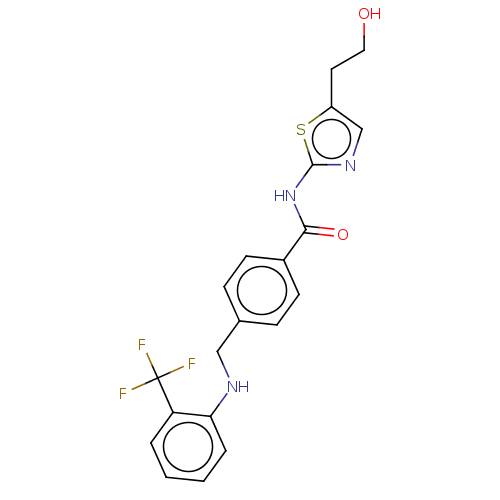
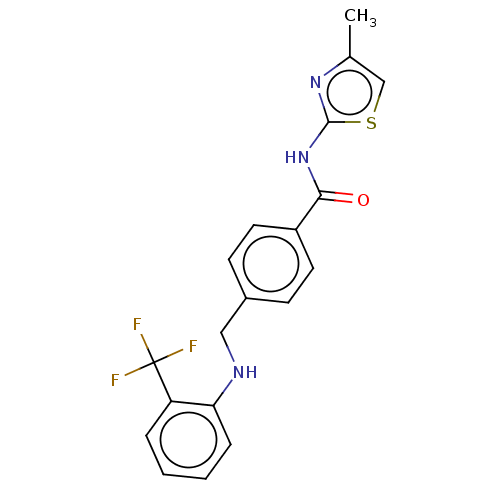
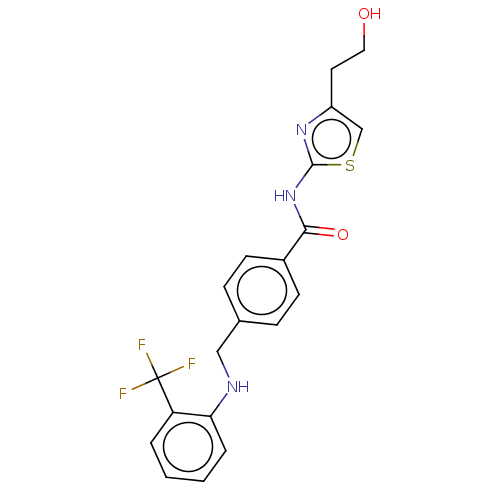
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.20nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 6.5nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Amgen, Inc.
Curated by ChEMBL
Amgen, Inc.
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 37nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 47nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 51nMAssay Description:Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNAD-dependent protein deacetylase sirtuin-2(Homo sapiens (Human))
Kyoto Prefectural University of Medicine
Curated by ChEMBL
Kyoto Prefectural University of Medicine
Curated by ChEMBL
Affinity DataKi: 68nMAssay Description:Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu...More data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 80nMAssay Description:Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 82nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 86nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 89nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 103nMAssay Description:Inhibition of [3H]diprenorphine (0.33 nM) binding from human Opioid receptor mu 1 expressed in CHO-K1 cells.More data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 121nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNociceptin receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 369nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNAD-dependent protein deacetylase sirtuin-2(Homo sapiens (Human))
Kyoto Prefectural University of Medicine
Curated by ChEMBL
Kyoto Prefectural University of Medicine
Curated by ChEMBL
Affinity DataKi: 470nMAssay Description:Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu...More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.06E+3nMAssay Description:Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor kappa 1More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 8.65E+3nMAssay Description:Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor delta 1 expressed in CHO-K1 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 0.980nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Inhibition of mouse liver microsome SCD assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]CoA as substrate in presence of NADH incu...More data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp...More data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Inhibition of recombinant human SCD1 expressed in baculovirus expression system assessed as reduction in [3H]H2O production using stearoyl [9,10-3H]C...More data for this Ligand-Target Pair

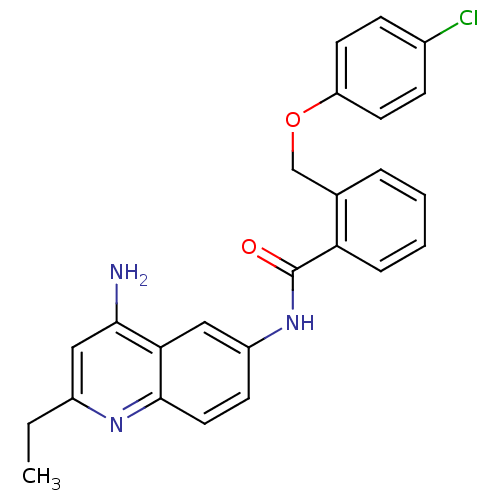


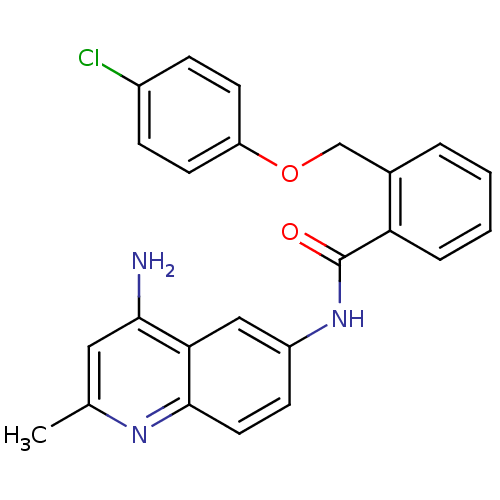
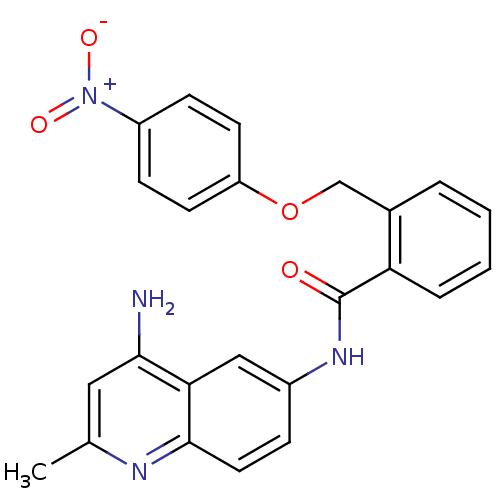


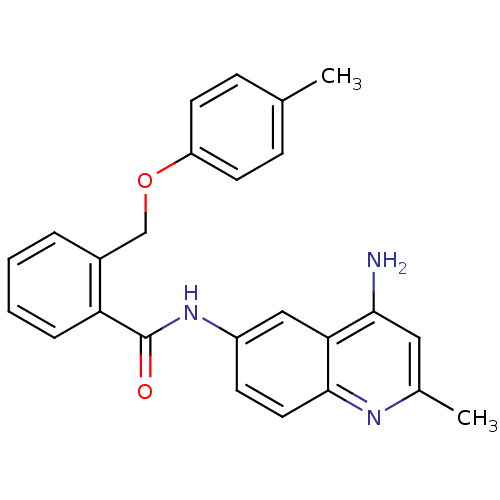
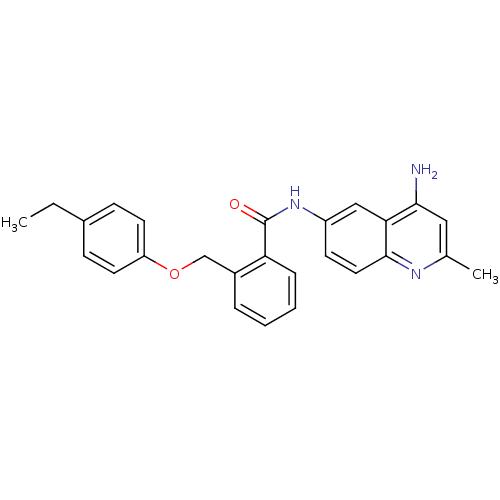

 3D Structure (crystal)
3D Structure (crystal)