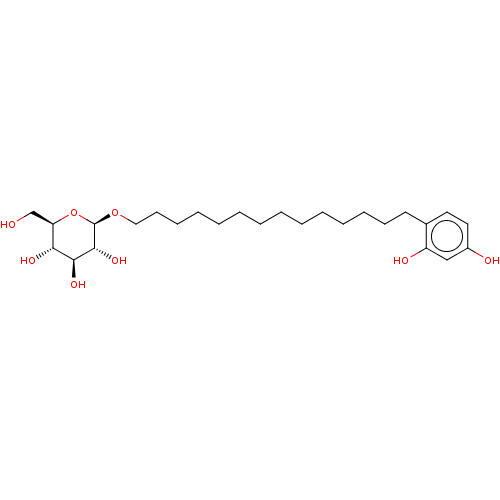
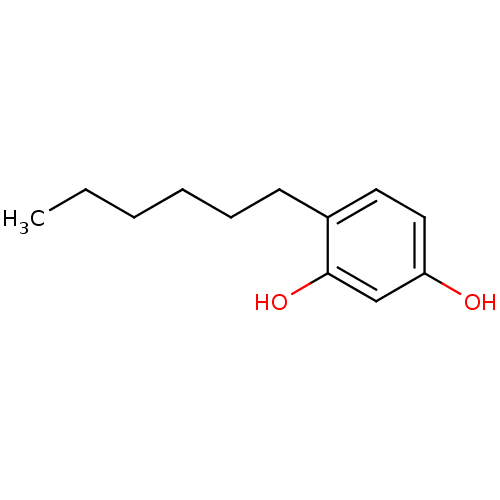
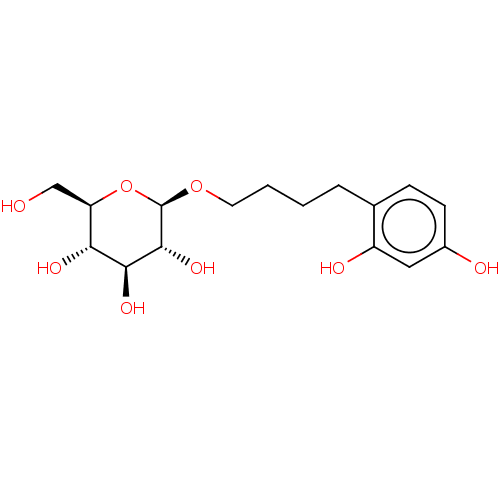
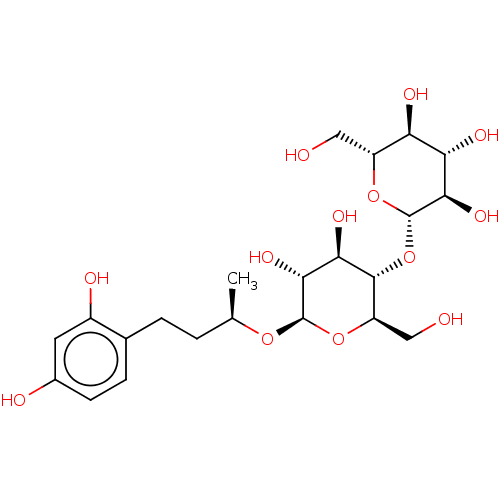
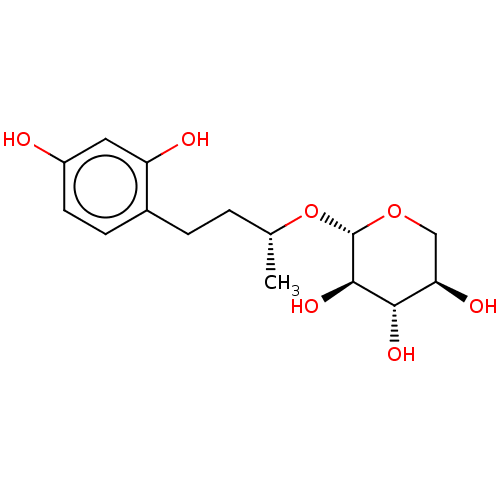
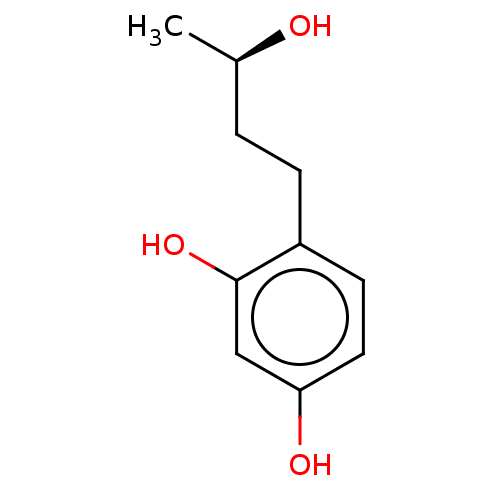
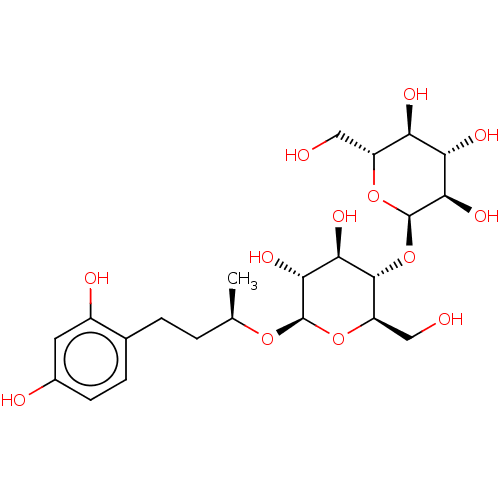
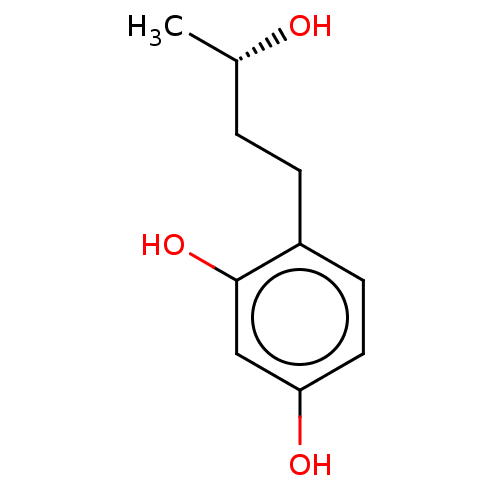
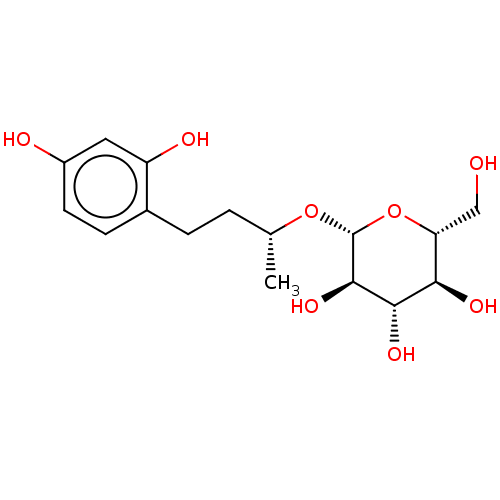
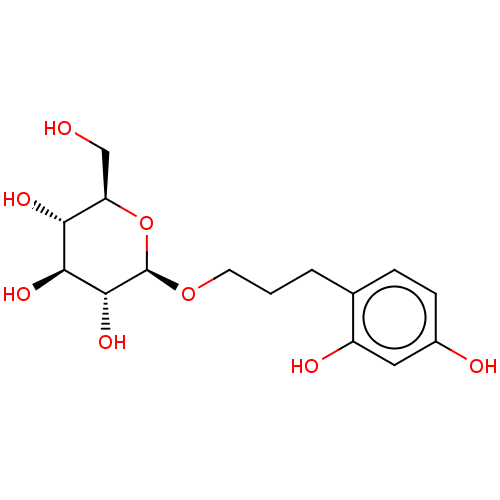
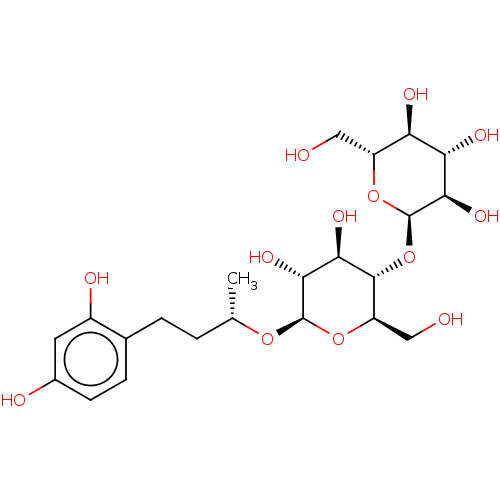
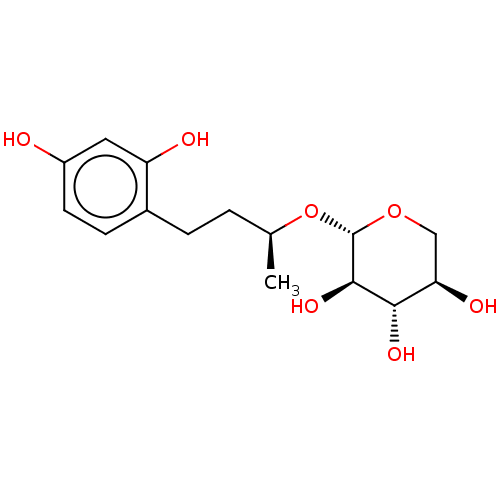
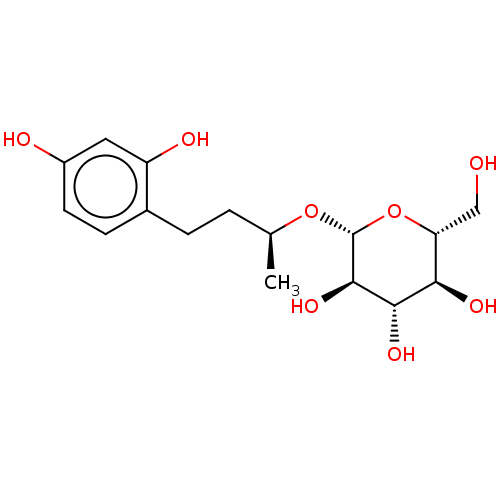
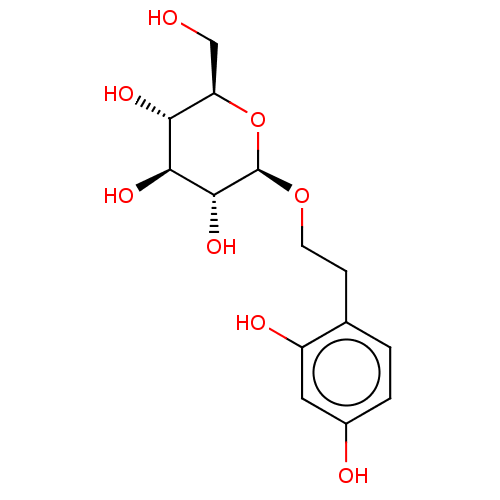
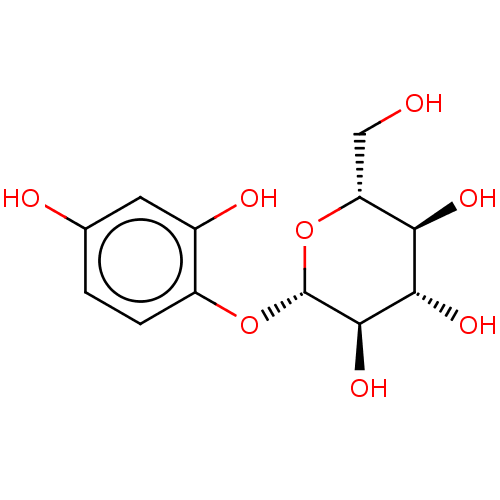
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 390nMAssay Description:Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 410nMAssay Description:Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 560nMAssay Description:Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 560nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 610nMAssay Description:Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 630nMAssay Description:Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 1.36E+3nMAssay Description:Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 1.51E+3nMAssay Description:Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 1.51E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 1.72E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 1.72E+3nMAssay Description:Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 1.78E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 1.98E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 2.17E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 3.62E+3nMAssay Description:Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 3.83E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 3.83E+3nMAssay Description:Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 4.13E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 4.56E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 4.56E+3nMAssay Description:Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 4.72E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 4.72E+3nMAssay Description:Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 9.15E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 9.15E+3nMAssay Description:Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 1.07E+4nMAssay Description:Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Utsunomiya University
Curated by ChEMBL
Utsunomiya University
Curated by ChEMBL
Affinity DataIC50: 3.59E+4nMAssay Description:Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetTyrosinase(Homo sapiens (Human))
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Tokyo University of Agriculture and Technology
Curated by ChEMBL
Affinity DataIC50: 4.17E+5nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate preincubated with substrate for 5 mins followed by enzyme addition and measured i...More data for this Ligand-Target Pair