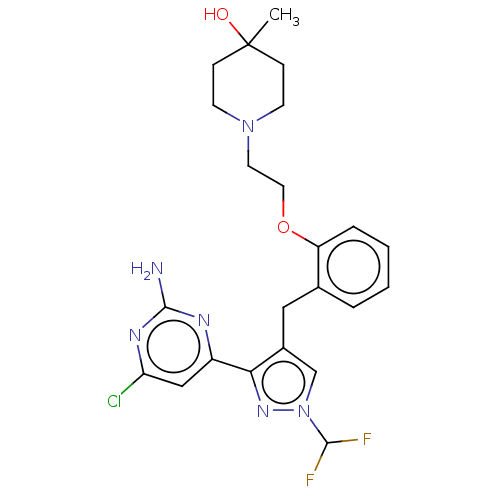
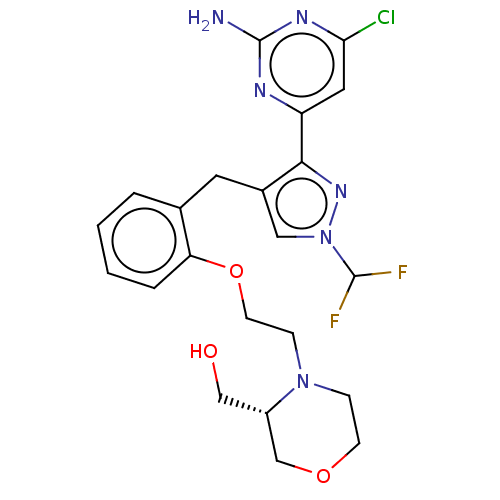
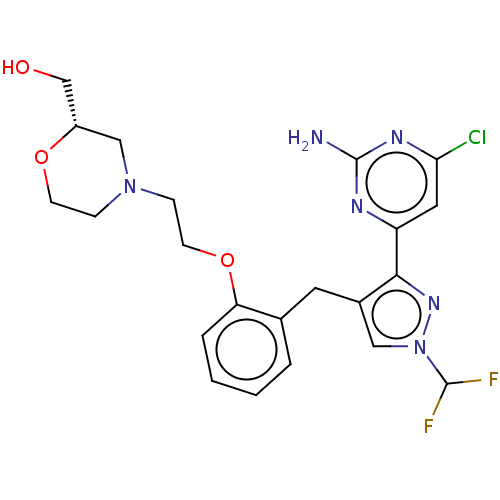
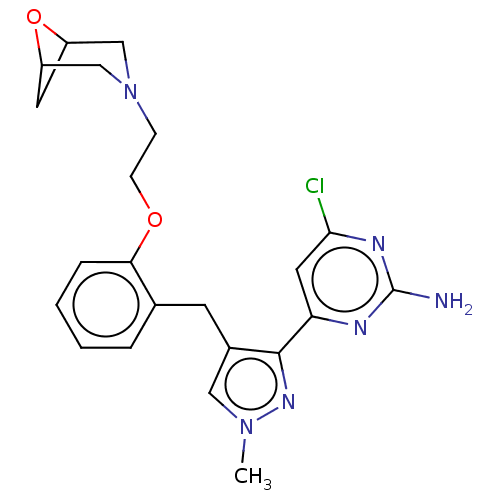
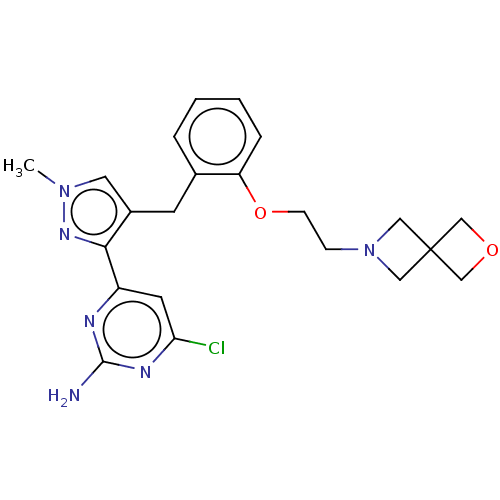
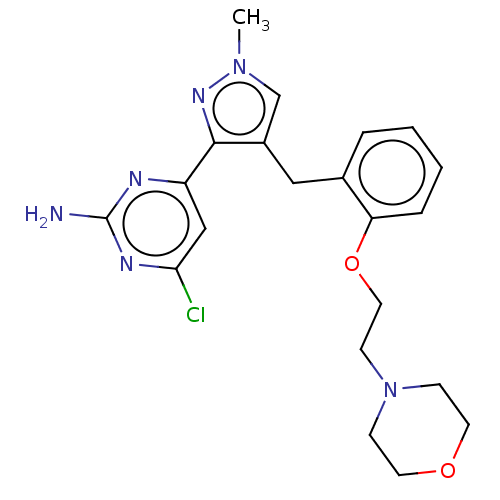
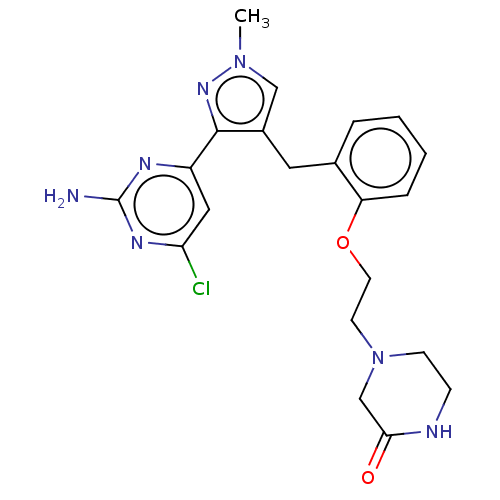
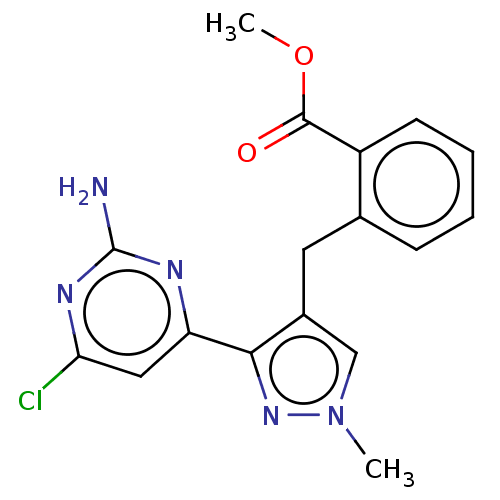
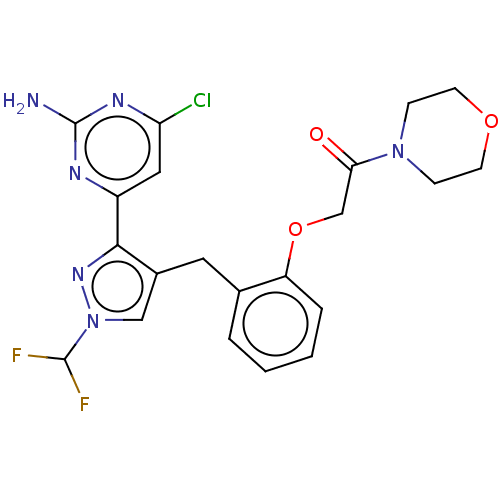
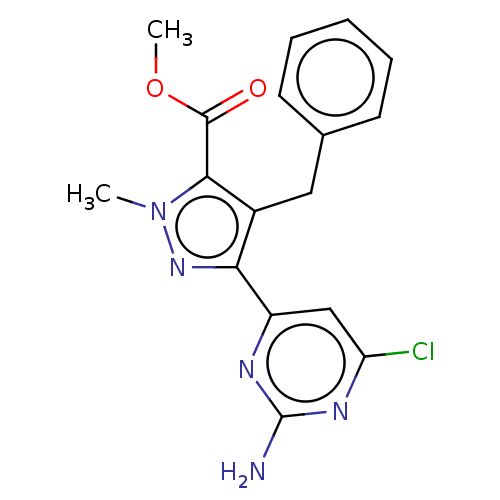
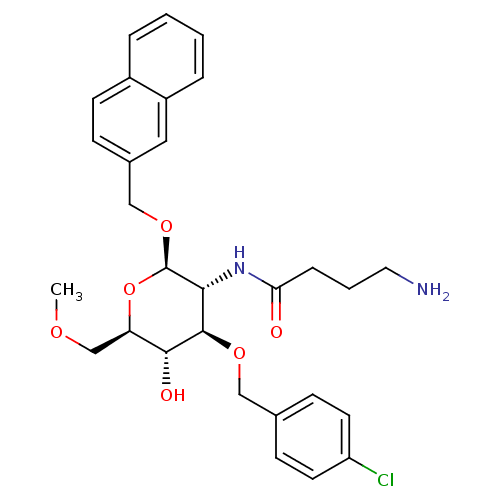
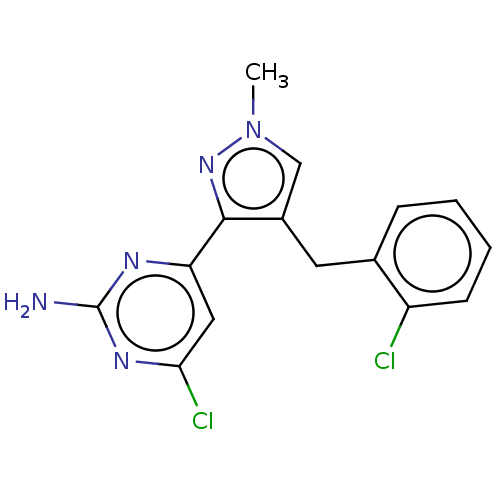
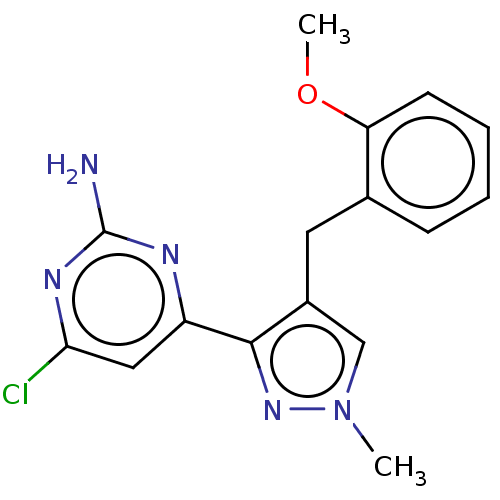
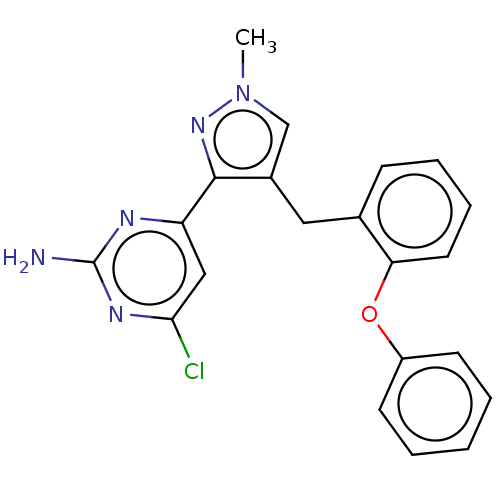
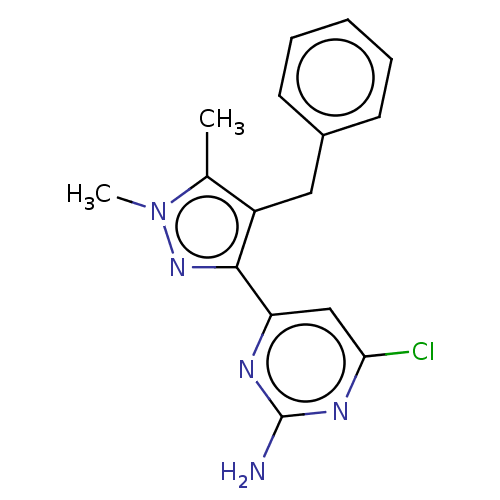
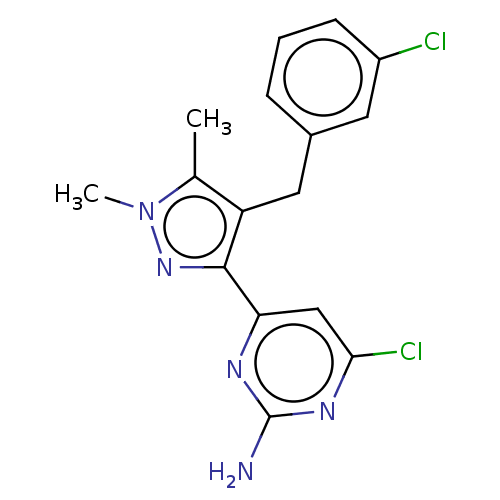
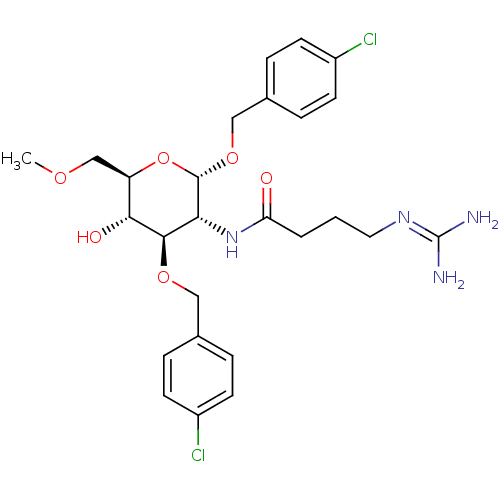
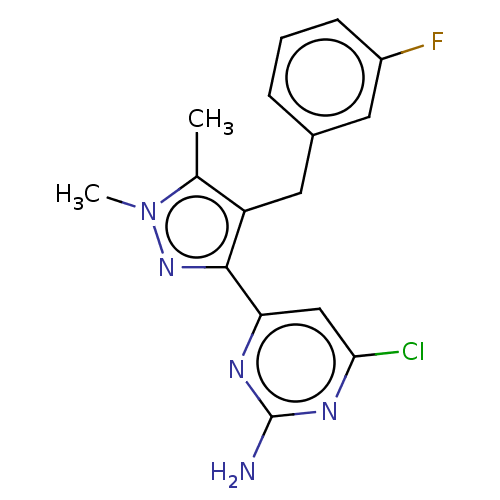
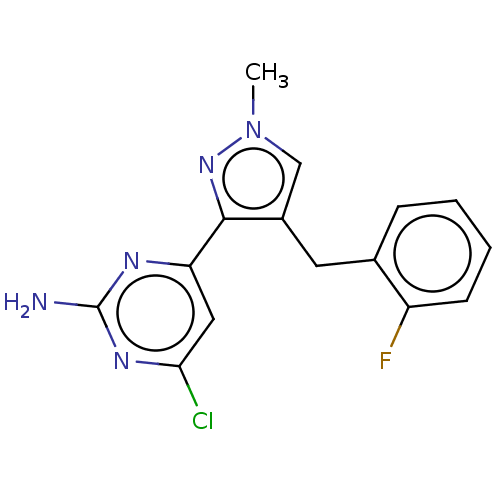
Affinity DataKi: 1.20E+3nMAssay Description:Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation countingMore data for this Ligand-Target Pair
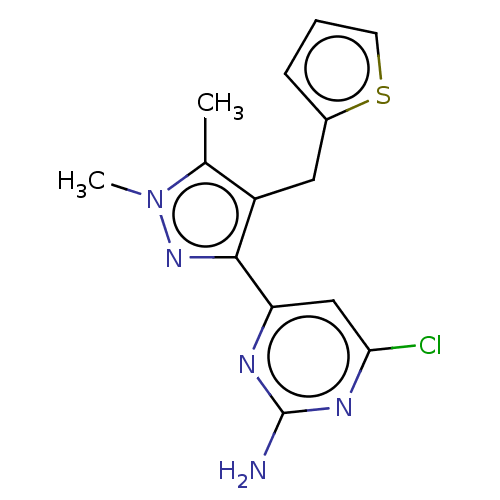
Affinity DataKi: 1.80E+3nMAssay Description:Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.60E+3nMAssay Description:Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3.50E+3nMAssay Description:Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 5.10E+3nMAssay Description:Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 6.30E+3nMAssay Description:Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 6.60E+3nMAssay Description:Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation countingMore data for this Ligand-Target Pair
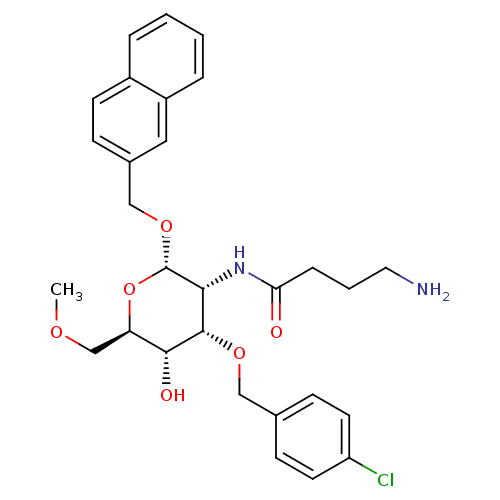
Affinity DataIC50: 28nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
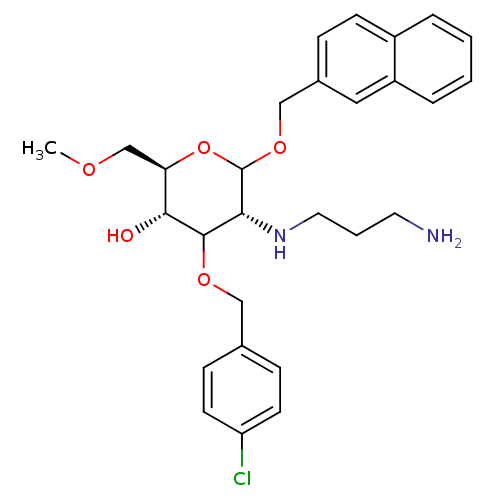
Affinity DataIC50: 50nMAssay Description:Displacement of [125I]iodotyrosyl from human SST5 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Displacement of [125I]iodotyrosyl from human SST5 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Displacement of [125I]iodotyrosyl from human SST5 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 74nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
Affinity DataIC50: 92nMAssay Description:Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Displacement of [125I]iodotyrosyl from human SST5 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 102nMAssay Description:Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 107nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
Affinity DataIC50: 132nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair

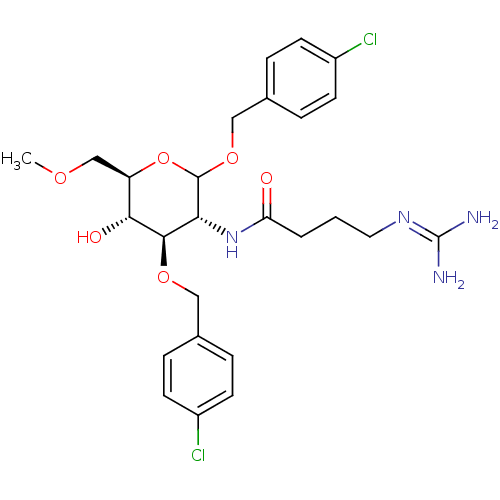
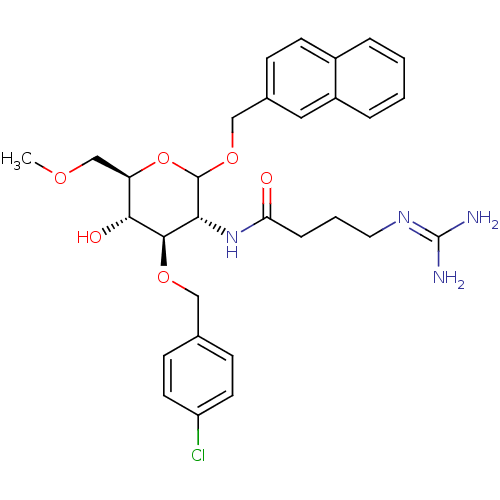

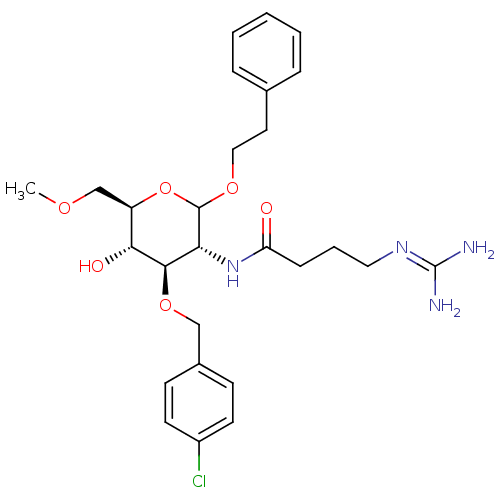
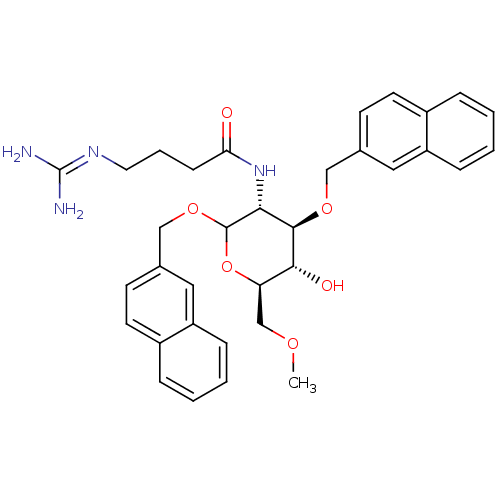
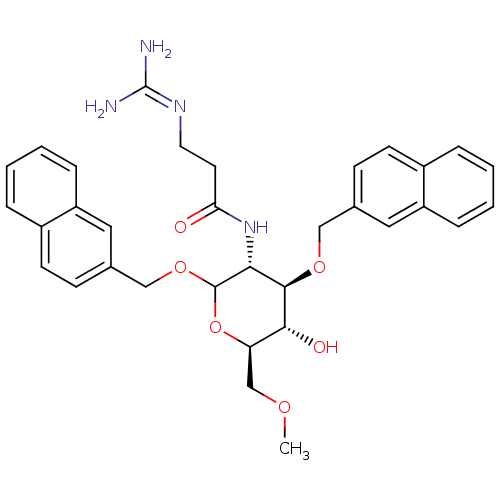
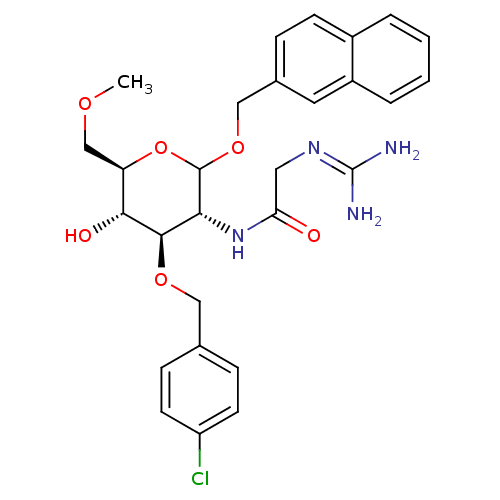
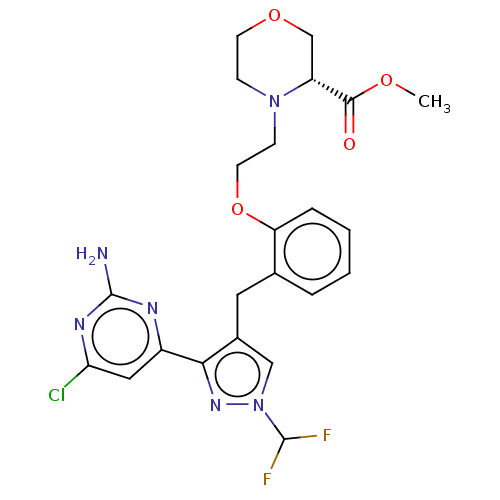
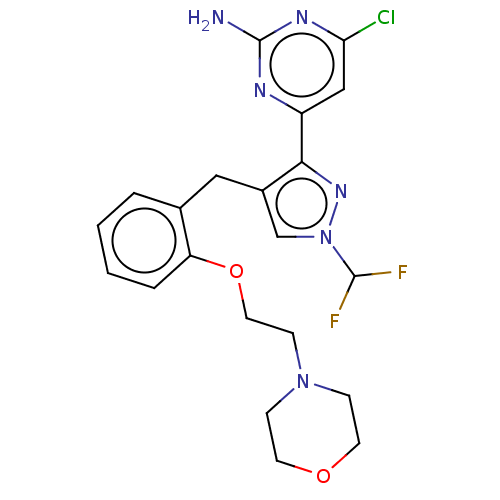

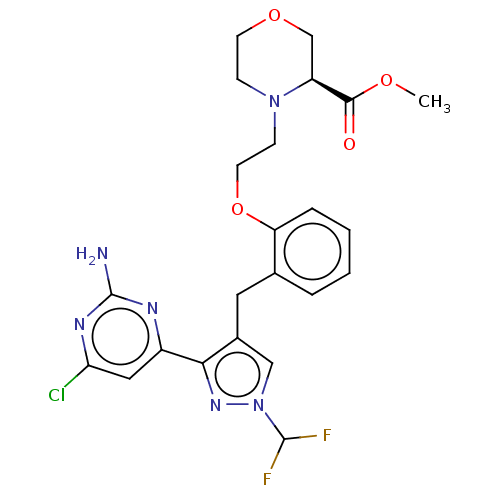
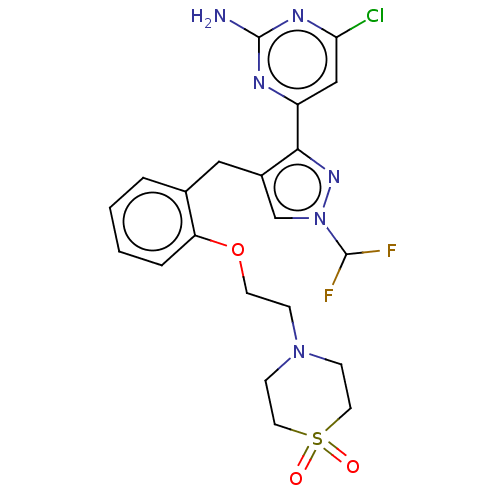
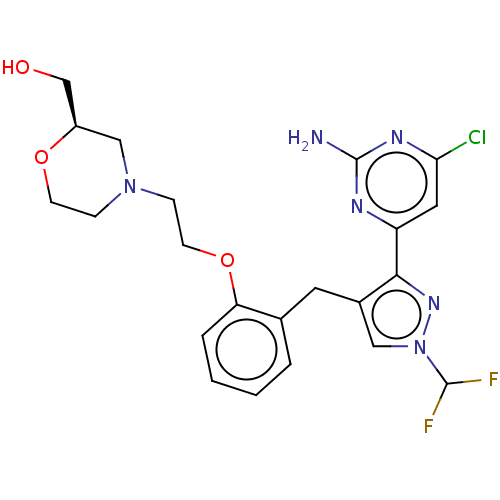
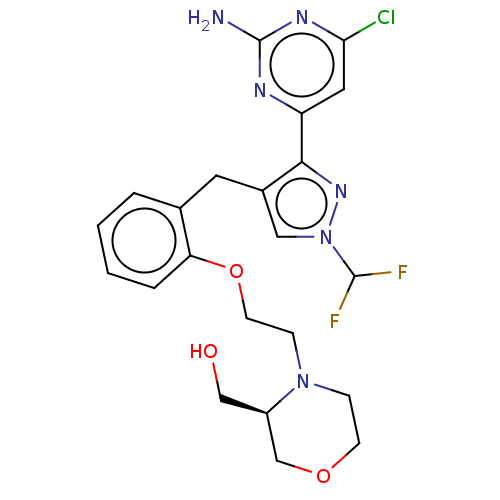
 3D Structure (crystal)
3D Structure (crystal)