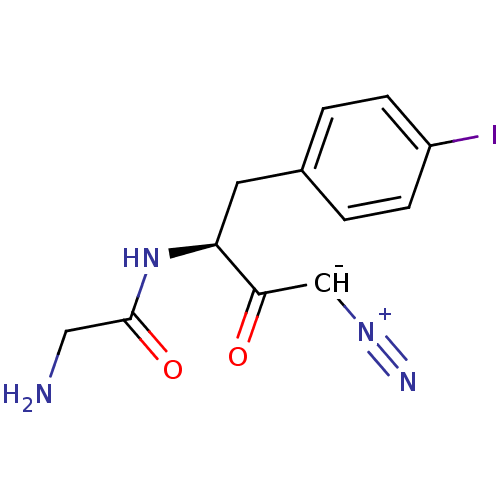
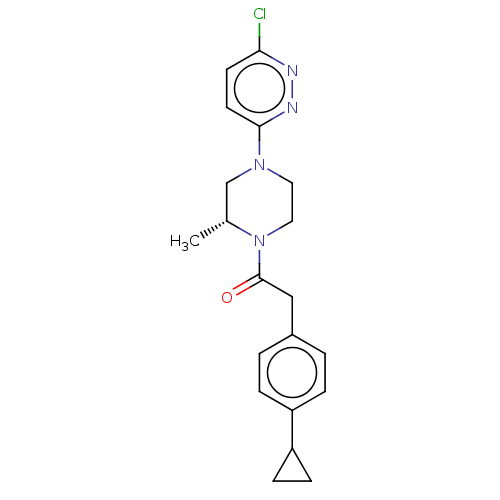
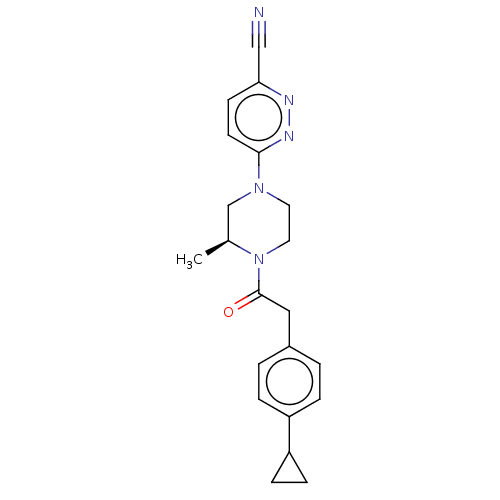
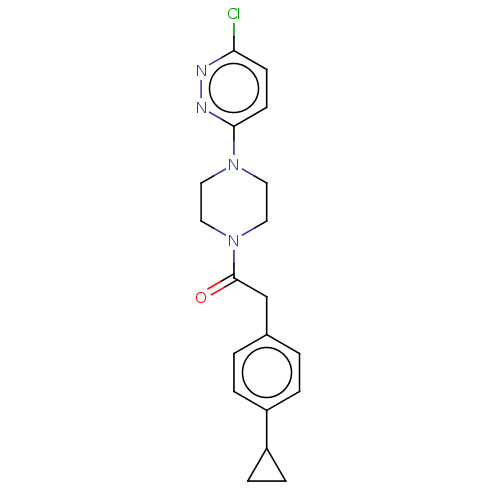
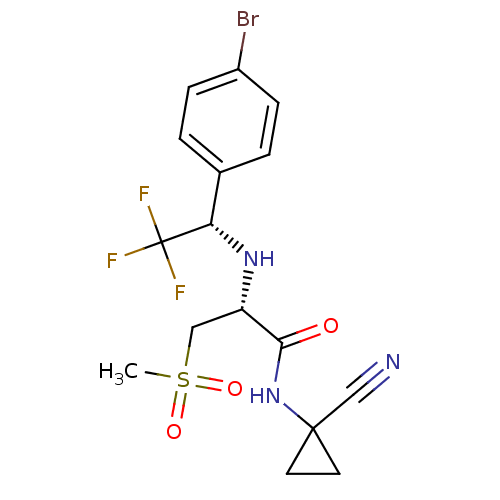
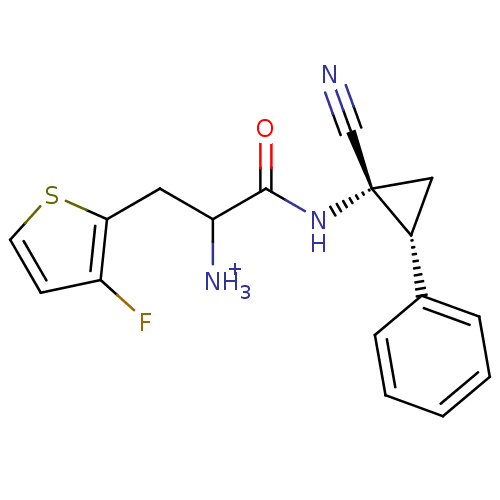
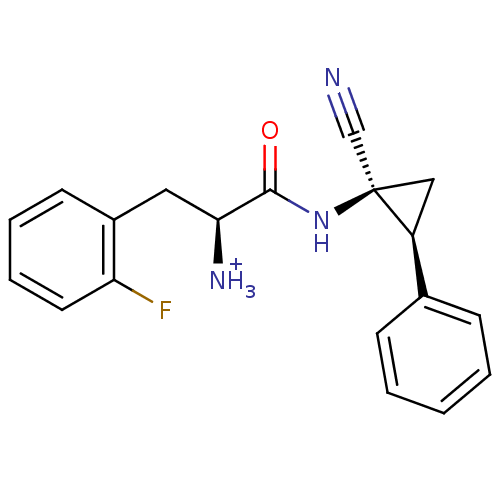
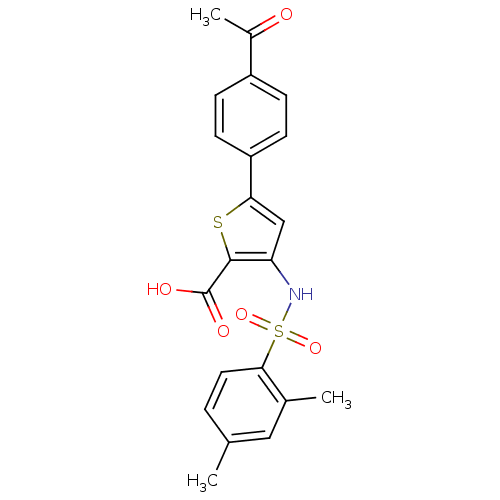
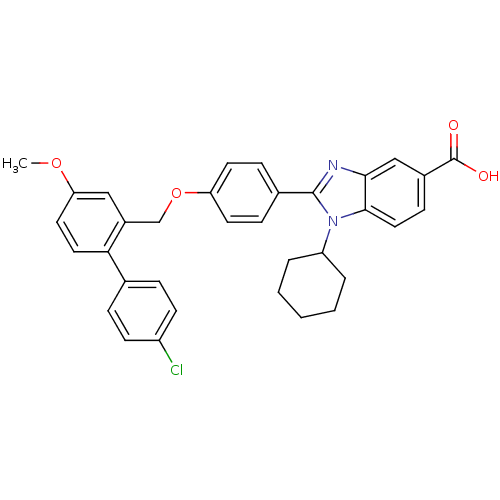
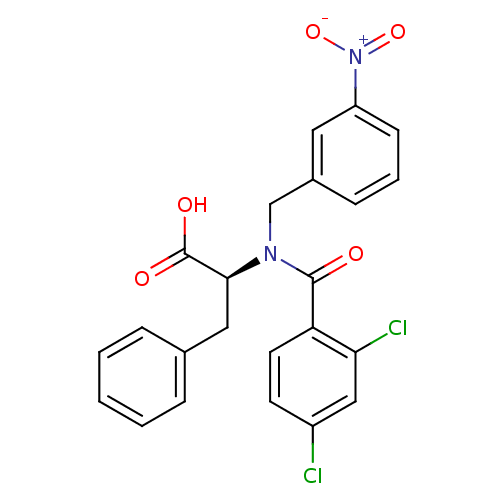
Affinity DataIC50: 1nMAssay Description:Inhibition of human recombinant cathepsin S after 10 minsMore data for this Ligand-Target Pair
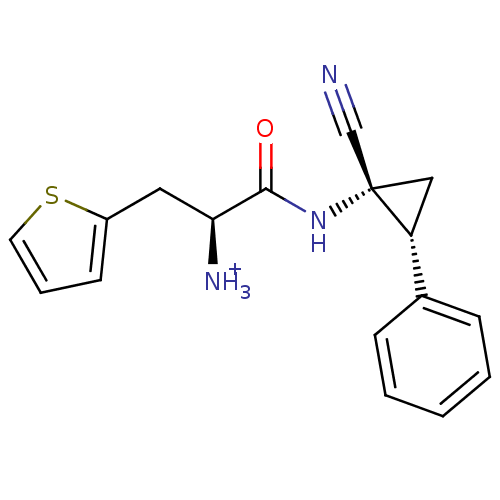
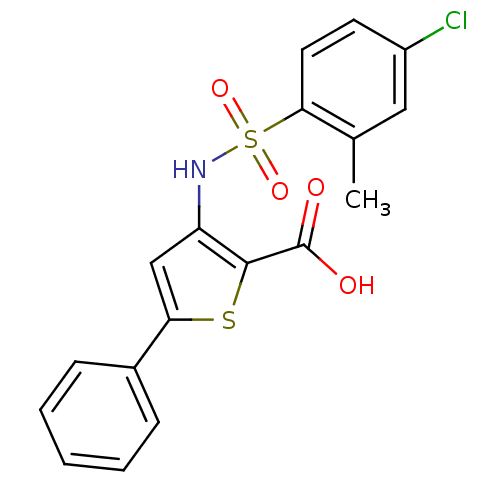
Affinity DataIC50: 1nMAssay Description:Inhibition of human recombinant cathepsin C after 10 minsMore data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
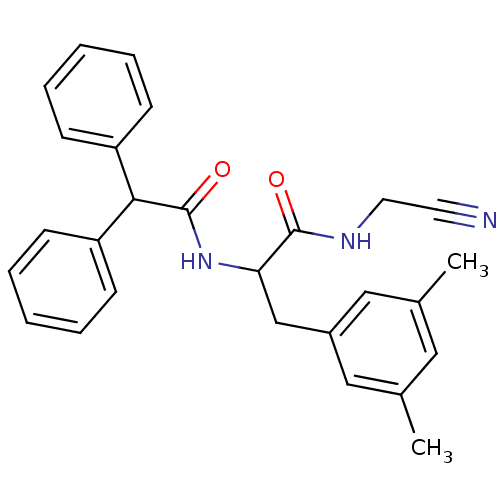
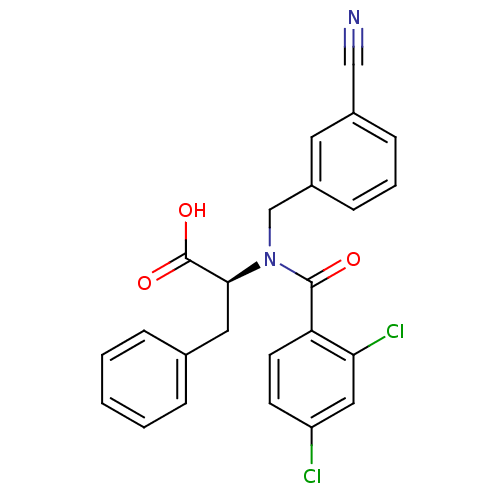
Affinity DataIC50: 7nMAssay Description:Inhibition of human recombinant cathepsin L after 10 minsMore data for this Ligand-Target Pair
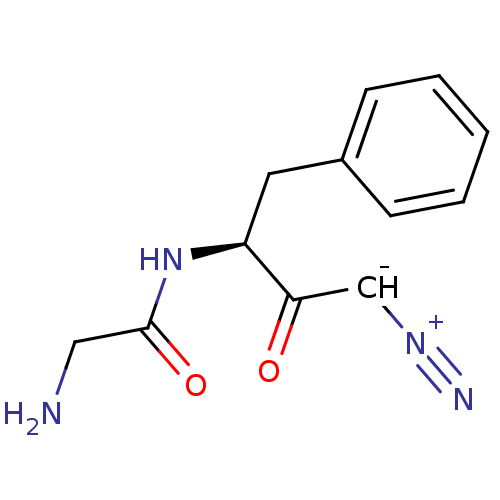
Affinity DataIC50: 7nMAssay Description:Inhibition of human recombinant cathepsin S after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human recombinant cathepsin S after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant cathepsin S after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant cathepsin B after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant cathepsin L after 10 minsMore data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
Affinity DataIC50: 13nMAssay Description:Inhibition of human recombinant cathepsin L after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant cathepsin C after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of human recombinant cathepsin B after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of human recombinant cathepsin C after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 65nMAssay Description:Inhibition of human recombinant cathepsin B after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Blockade of cathepsin G processing in human U937 cells by densitometryMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of human recombinant cathepsin L after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:Inhibition of proteinase-3 activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Blockade of cathepsin G processing in human U937 cells by densitometryMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of proteinase-3 activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of neutrophil elastase activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of neutrophil elastase activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:Blockade of neutrophil elastase processing in human U937 cells after 7 days by fluorogenic substrate cleavage assayMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of human recombinant cathepsin H after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Blockade of neutrophil elastase processing in human U937 cells after 7 days by fluorogenic substrate cleavage assayMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibition of human recombinant cathepsin C after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Inhibition of human recombinant cathepsin B after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 390nMAssay Description:Inhibition of HCV NS5B polymeraseMore data for this Ligand-Target Pair
Affinity DataIC50: 430nMAssay Description:Inhibition of human recombinant cathepsin L after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 450nMAssay Description:Inhibition of human recombinant cathepsin C after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Inhibition of human recombinant cathepsin S after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 480nMAssay Description:Inhibition of cathepsin G activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:Inhibition of human recombinant cathepsin L after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 620nMAssay Description:Inhibitory activity against Hepatitis C RNA dependent RNA polymerase Nonstructural protein 5B (NS5B polymerase) expressed from baculovirus-infected S...More data for this Ligand-Target Pair
Affinity DataIC50: 690nMAssay Description:Inhibition of cathepsin G activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibitory activity against Hepatitis C RNA dependent RNA polymerase Nonstructural protein 5B (NS5B polymerase) expressed from baculovirus-infected S...More data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Inhibition of HCV NS5B polymeraseMore data for this Ligand-Target Pair
Affinity DataIC50: 770nMAssay Description:Inhibition of human recombinant cathepsin C after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 790nMAssay Description:Inhibition of human recombinant cathepsin C after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibitory activity against Hepatitis C RNA dependent RNA polymerase Nonstructural protein 5B (NS5B polymerase) expressed from baculovirus-infected S...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of HCV NS5B polymeraseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:In vitro inhibition of Hepatitis C polymerase.More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)