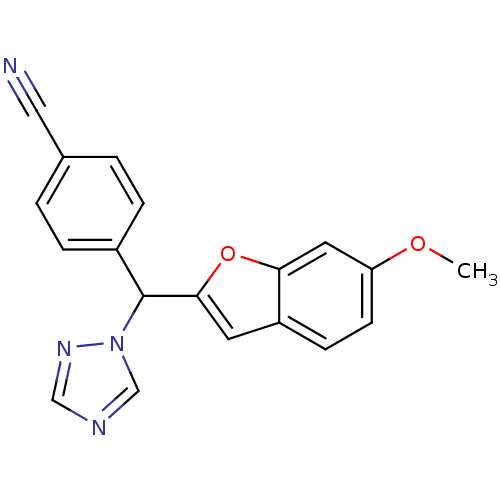
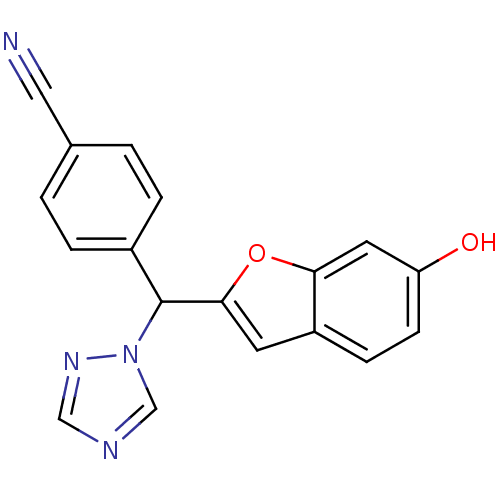
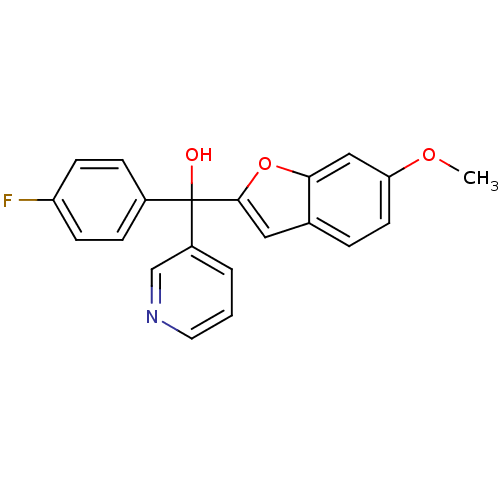
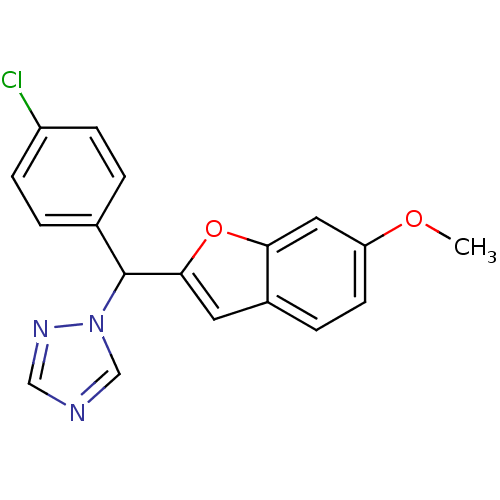
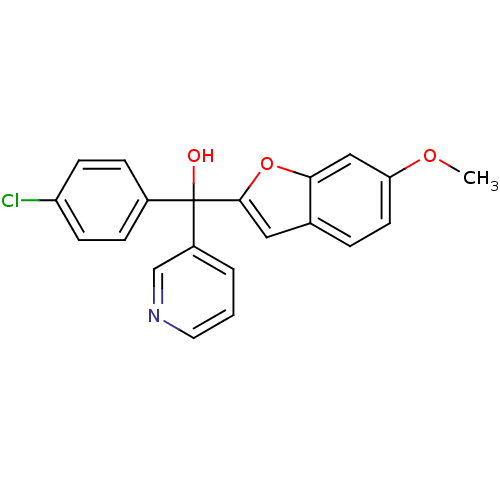
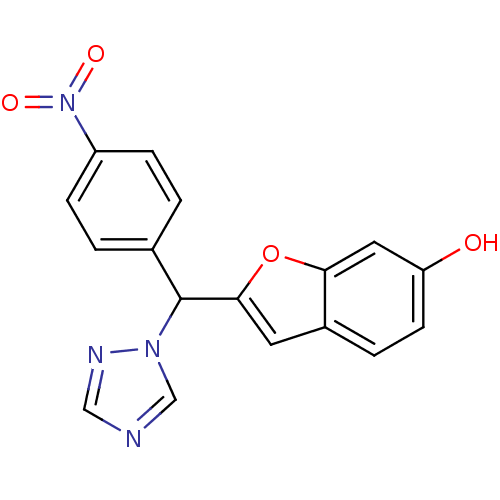
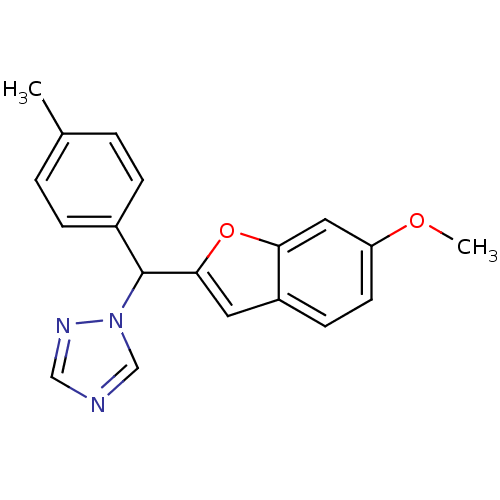
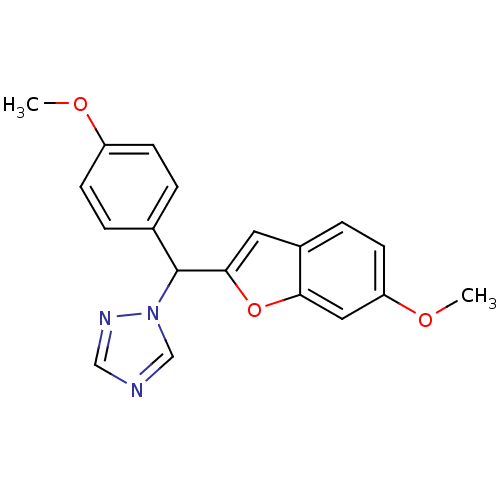
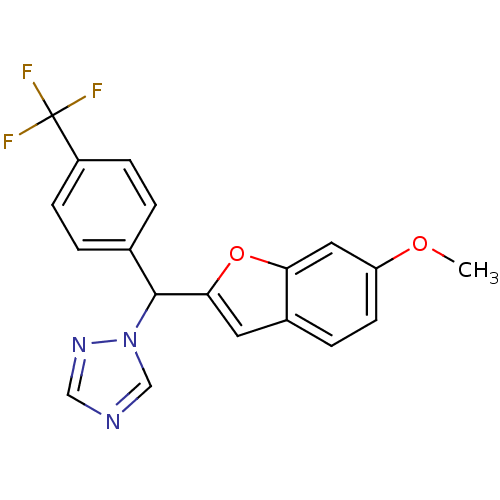
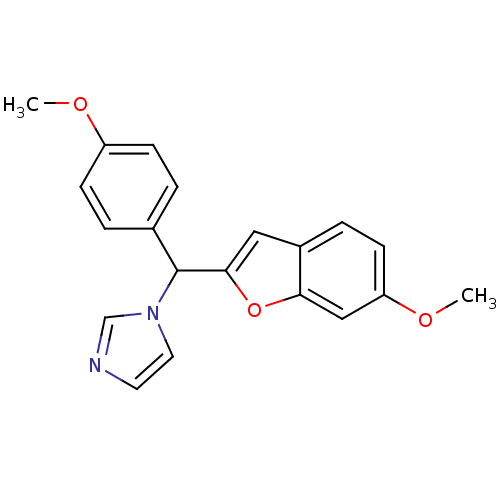
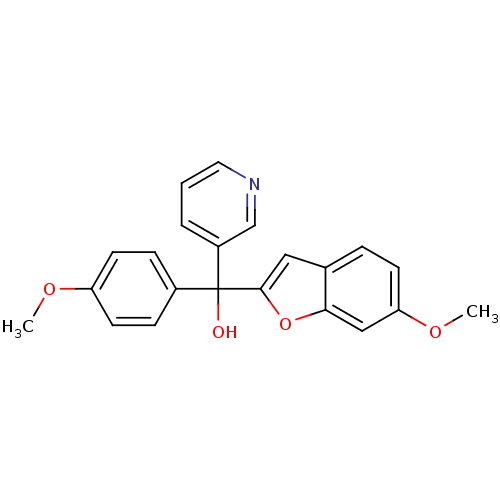
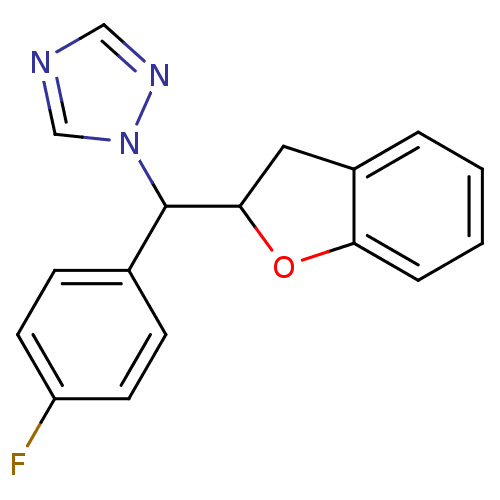
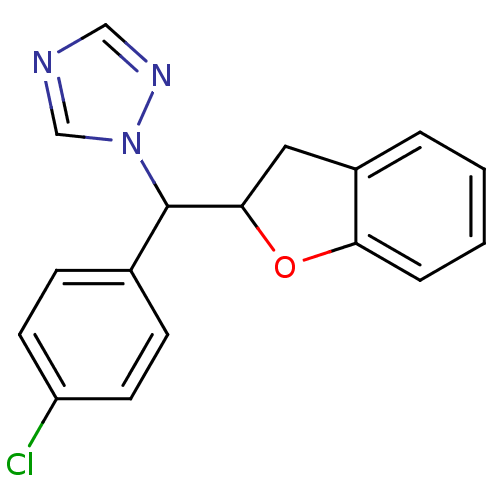
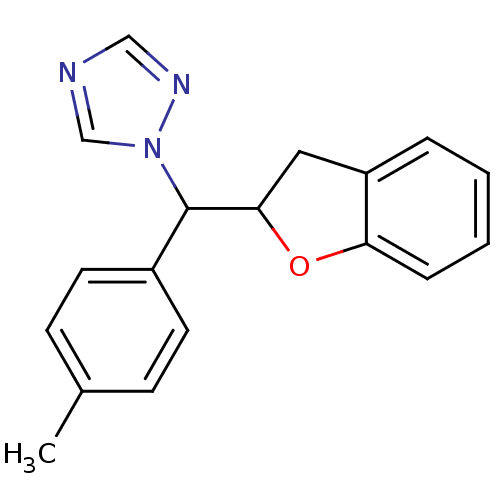
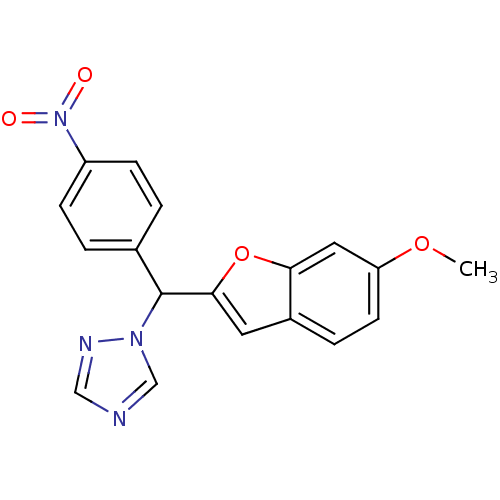
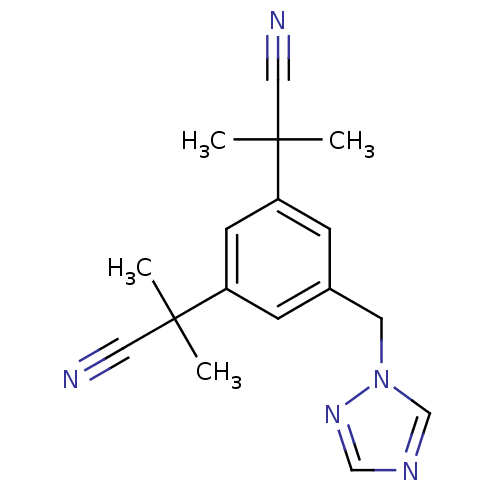
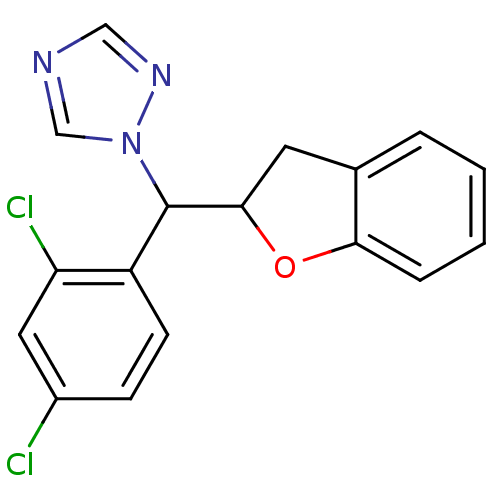
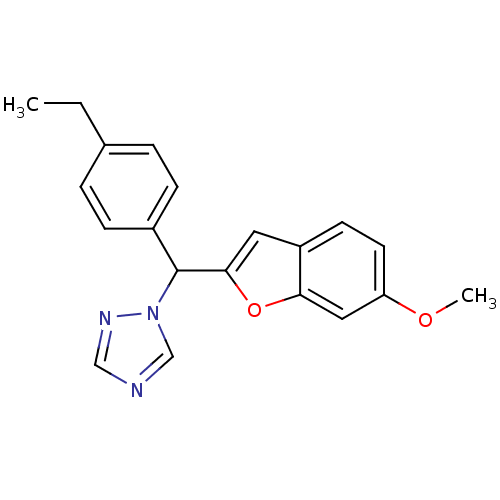
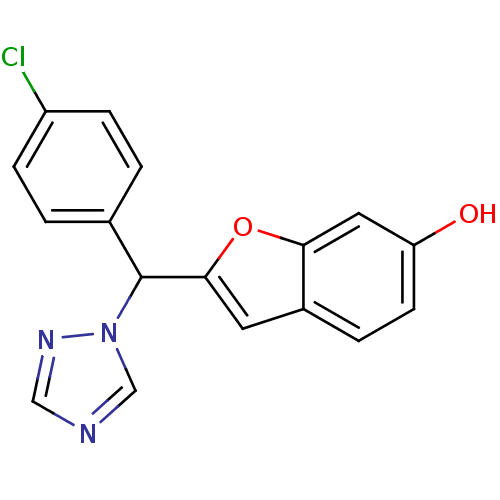
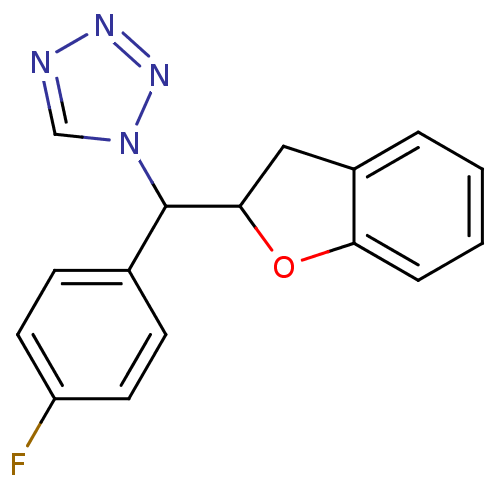
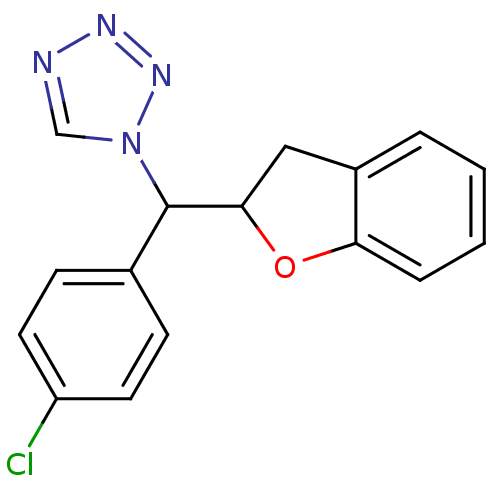
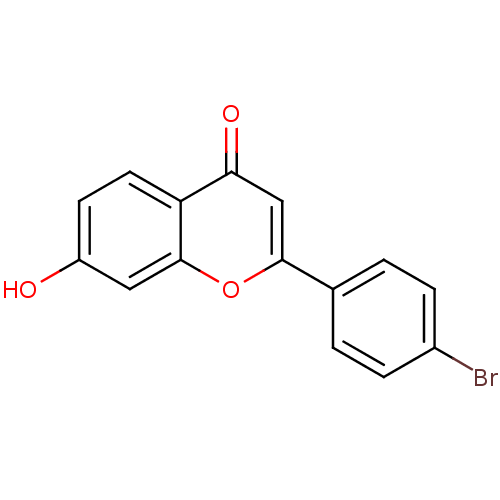
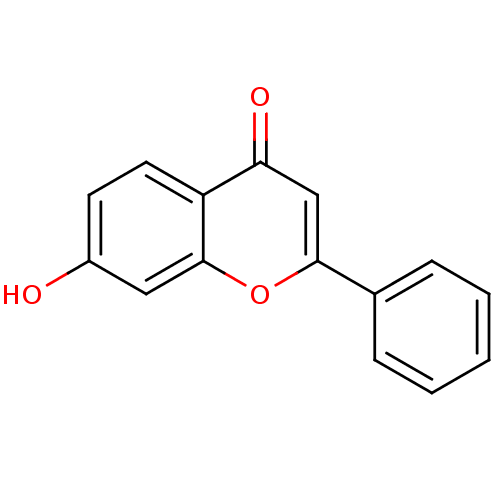
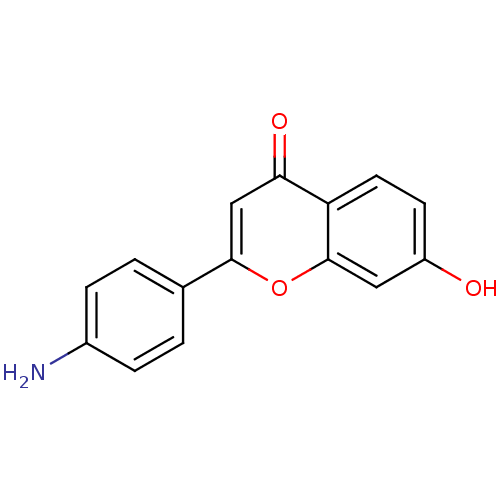
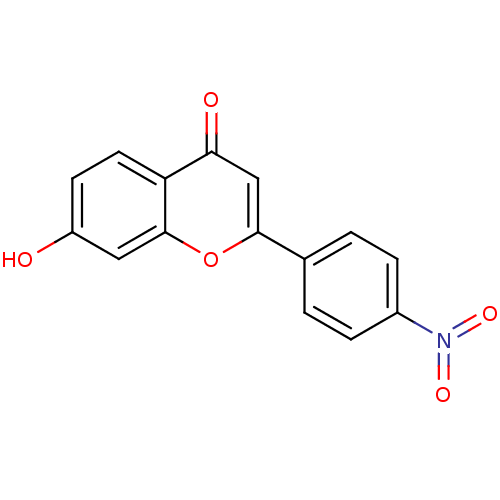
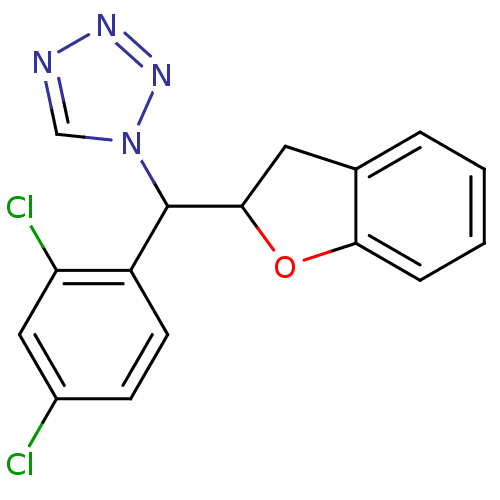
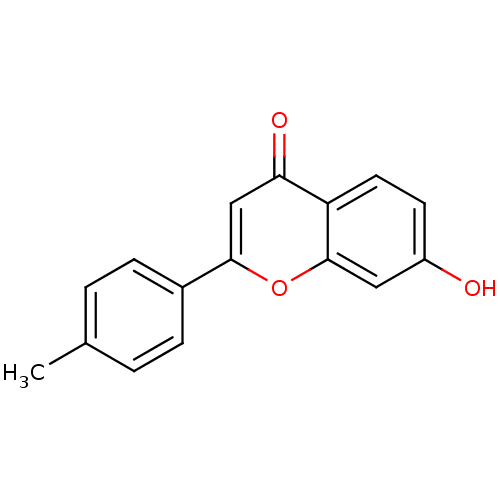
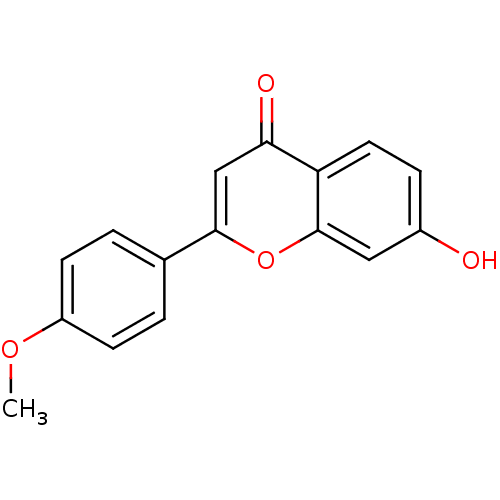
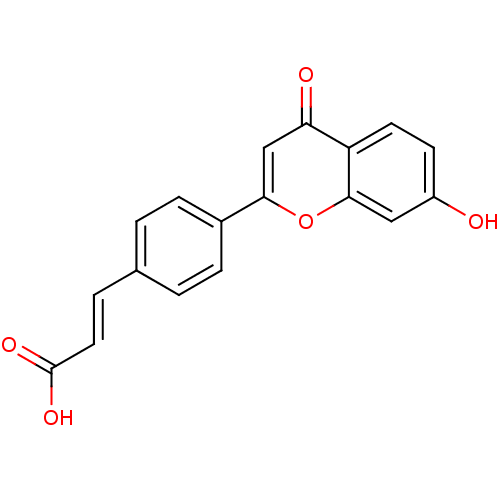
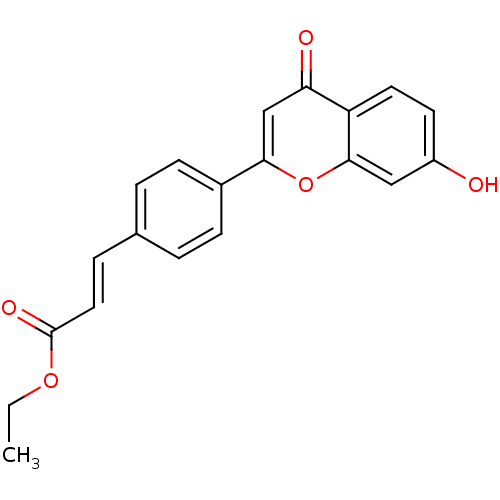
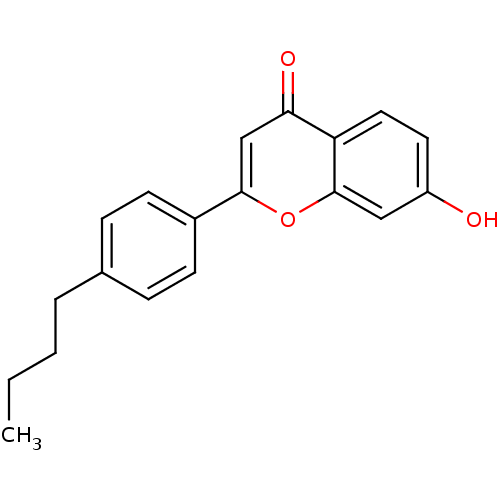
Affinity DataIC50: 10nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 49nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 49nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 130nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 130nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 130nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 160nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 590nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 600nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 880nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 1.23E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 1.46E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte...More data for this Ligand-Target Pair
Affinity DataIC50: 2.17E+3nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 1.01E+4nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.35E+4nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.72E+4nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+4nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+4nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair