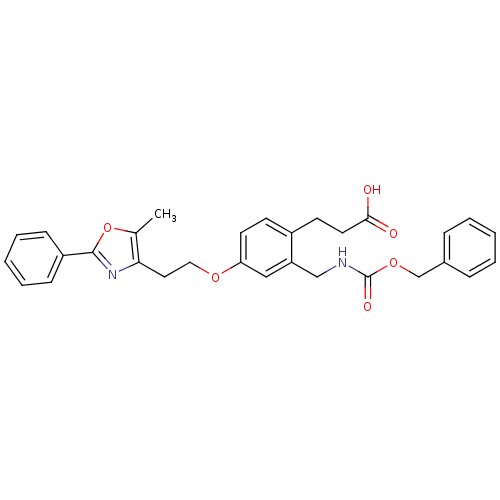
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 44nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 53nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 67nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 91nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 146nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 161nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 309nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 441nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 449nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 470nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 516nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 568nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
Ligand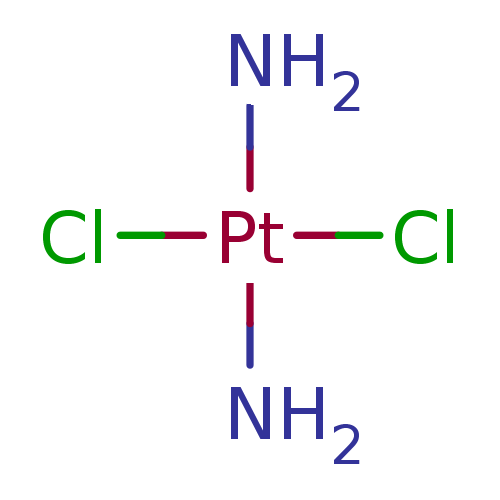 US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
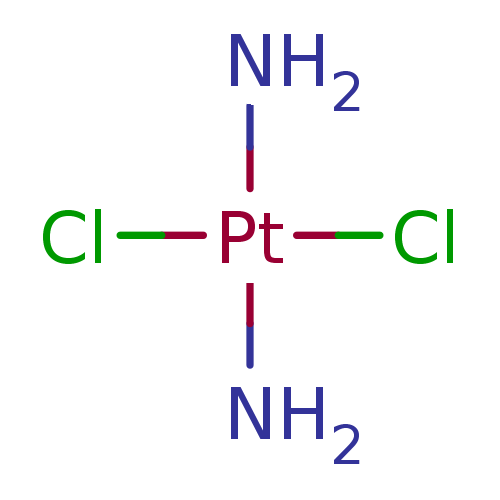 US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChITargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 706nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 770nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 1.15E+3nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 1.35E+3nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.39E+3nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor deltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.51E+3nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor deltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.57E+3nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor deltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 1.69E+3nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.92E+3nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor deltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.32E+3nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor deltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 2.71E+3nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
Ligand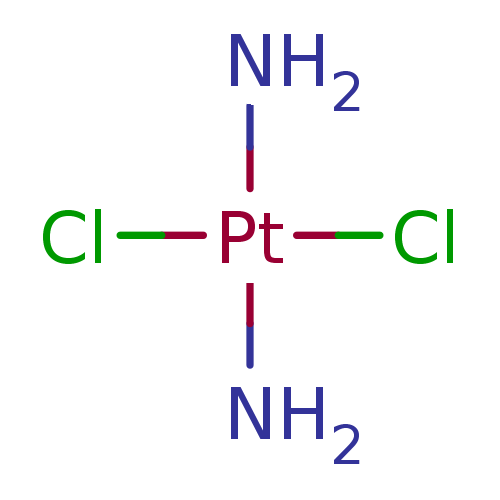 US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
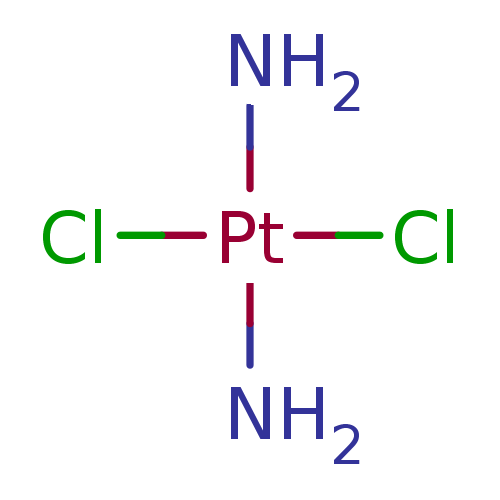 US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChIIn DepthDetails
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 3.39E+3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 3.49E+3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.94E+3nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor deltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.12E+3nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor deltaMore data for this Ligand-Target Pair
In DepthDetails
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 4.96E+3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
Ligand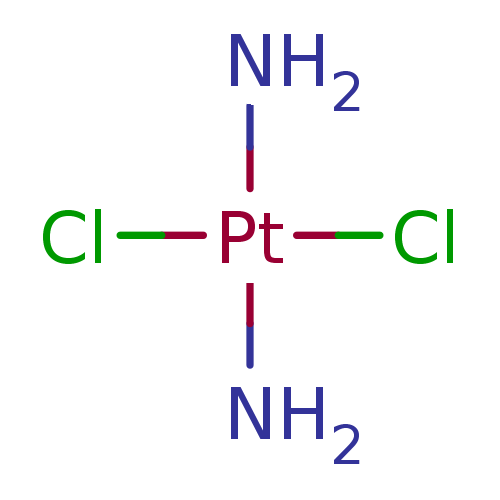 US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
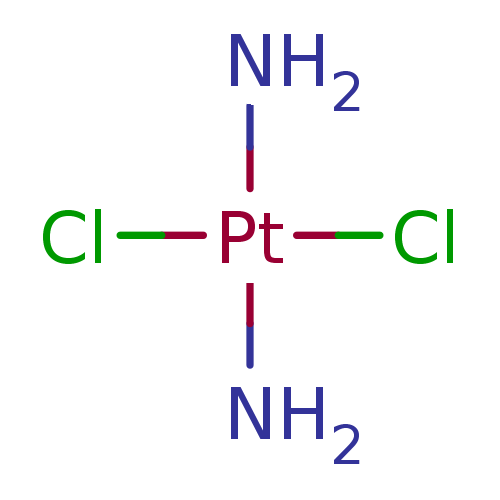 US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChIIn DepthDetails
Ligand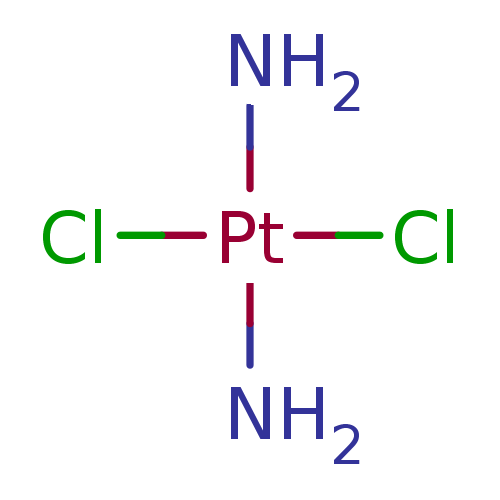 US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
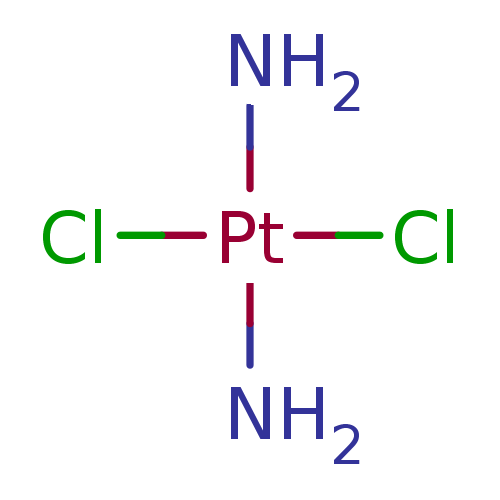 US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChI
US11952364, Example Control(Cis-Platinum(II) | Cisplatin(cis-Diammenedichlorop...)copy SMILEScopy InChIIn DepthDetails




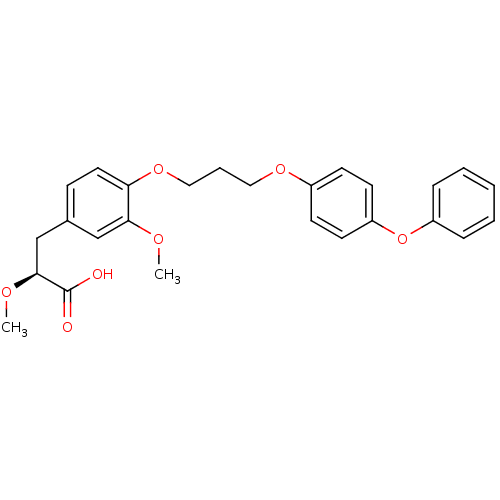



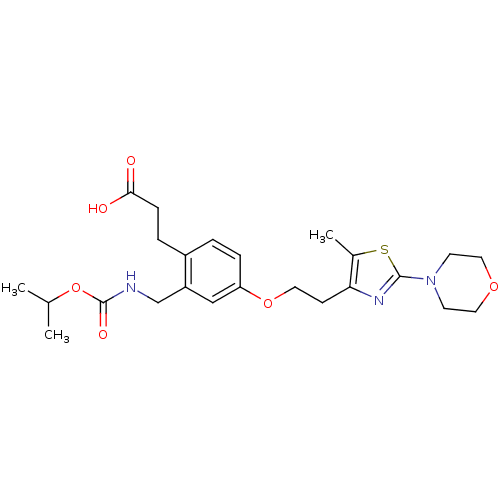




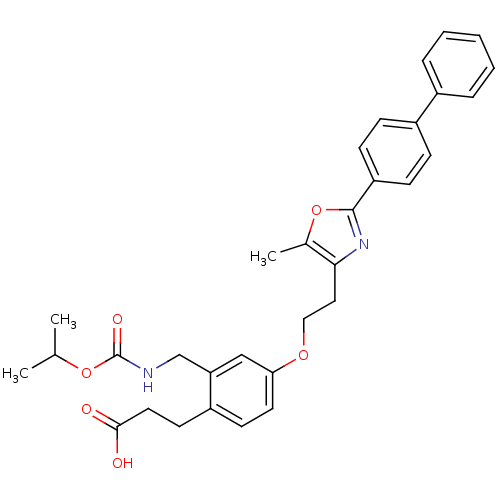


 3D Structure (crystal)
3D Structure (crystal)