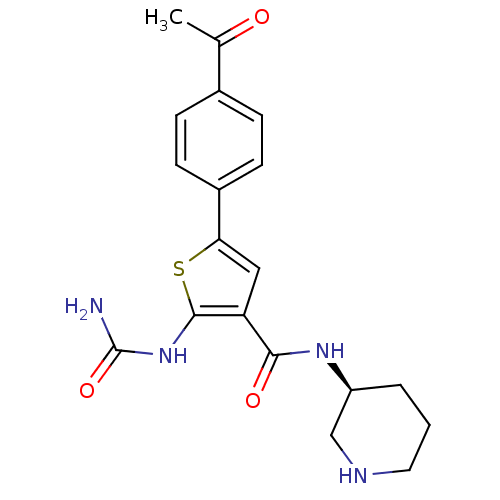
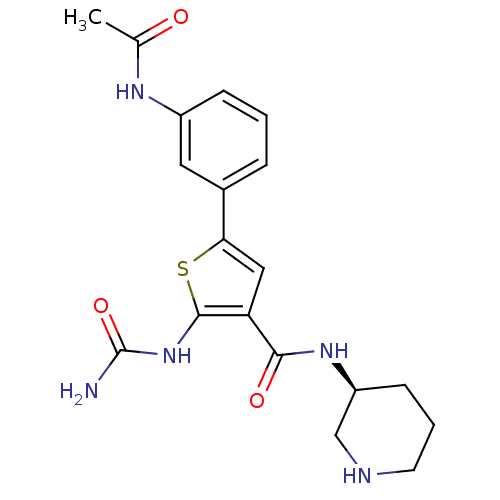
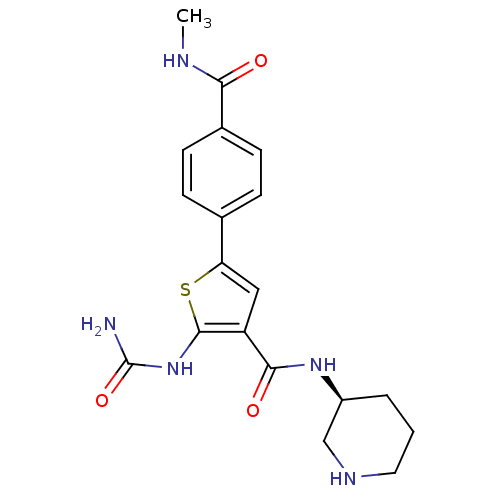
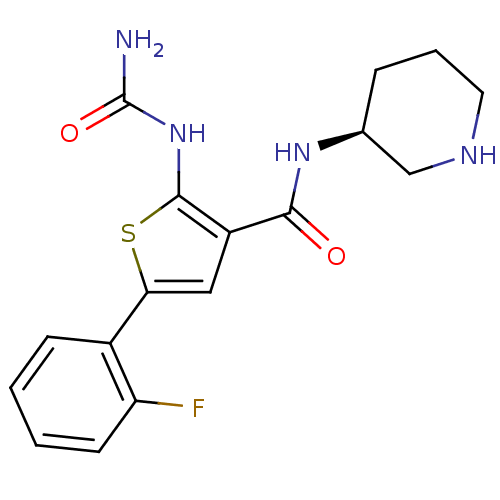
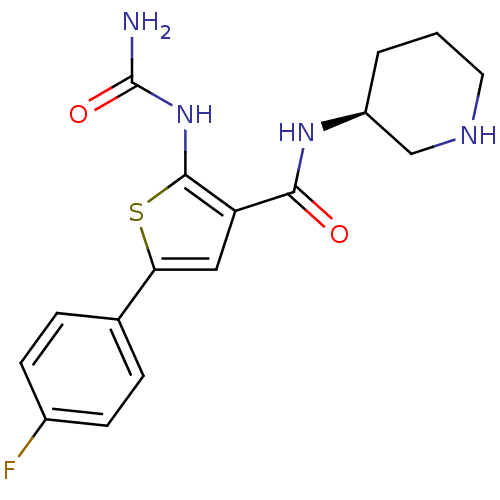
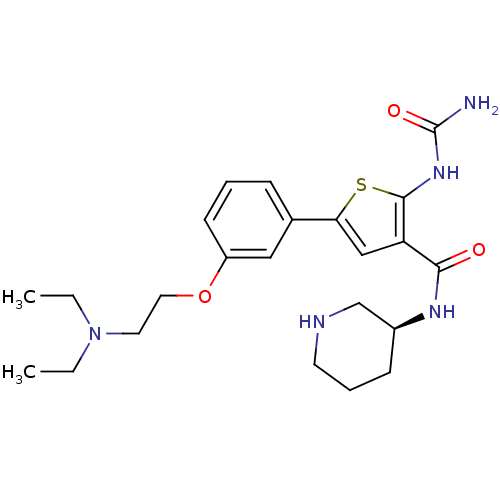
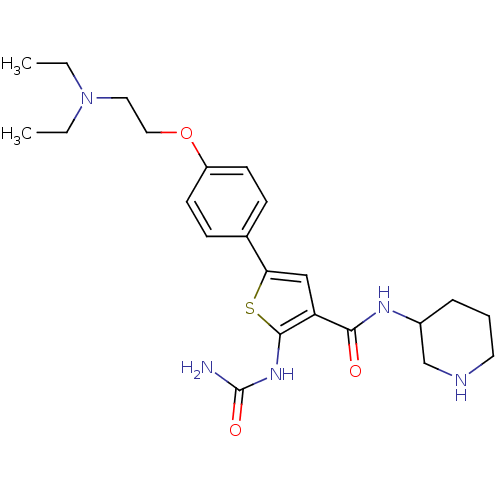
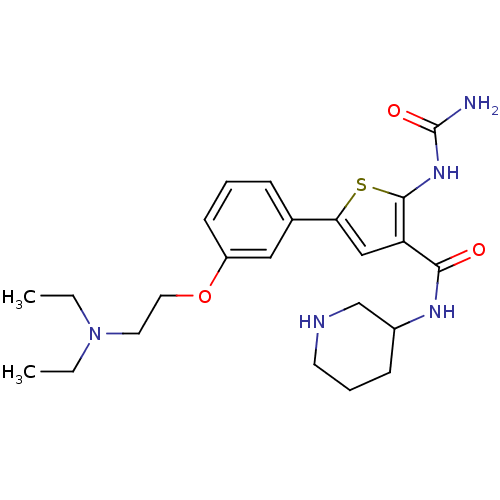
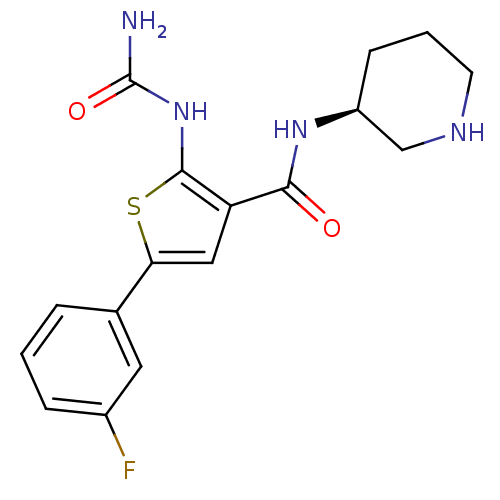
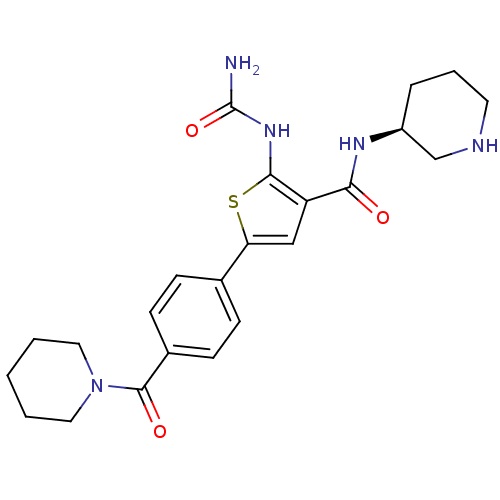
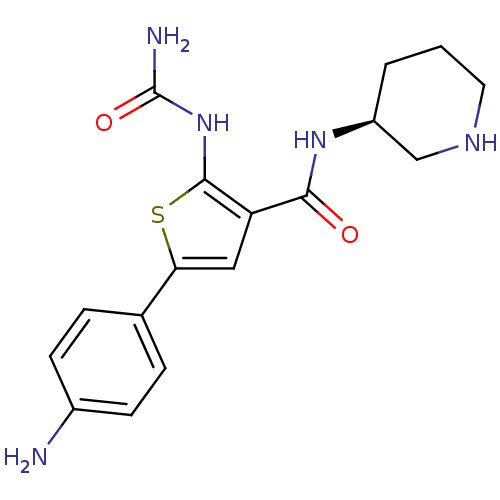
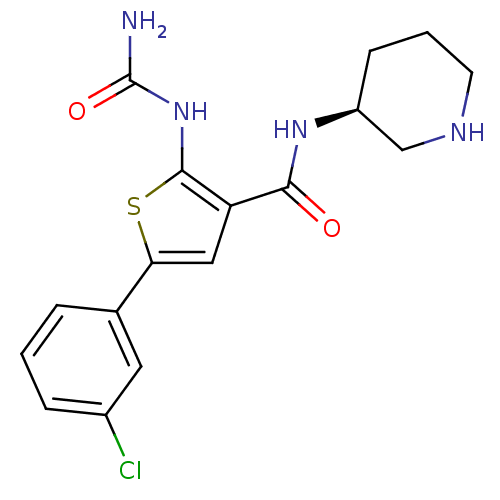
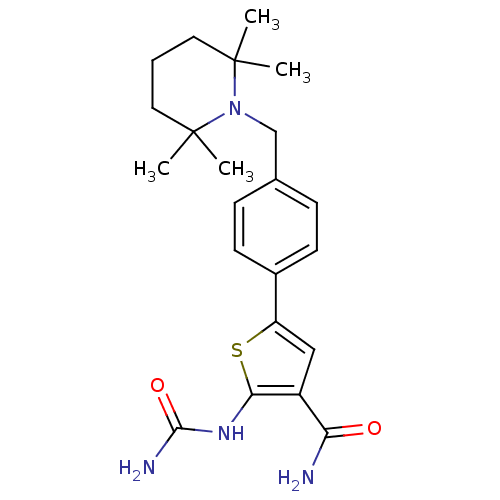
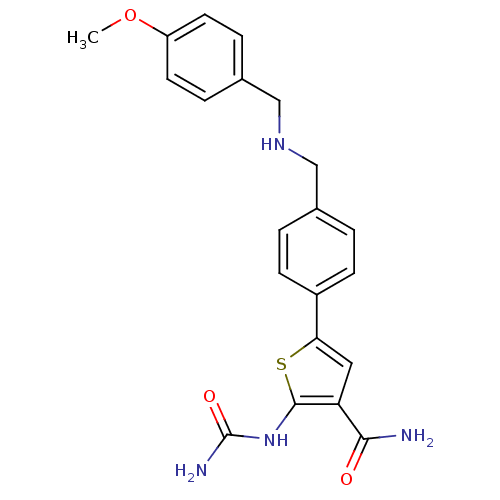
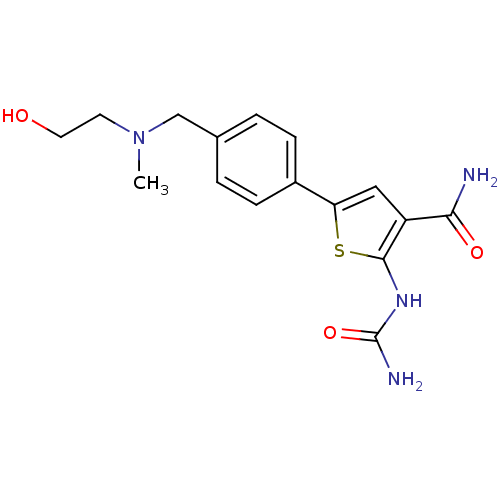
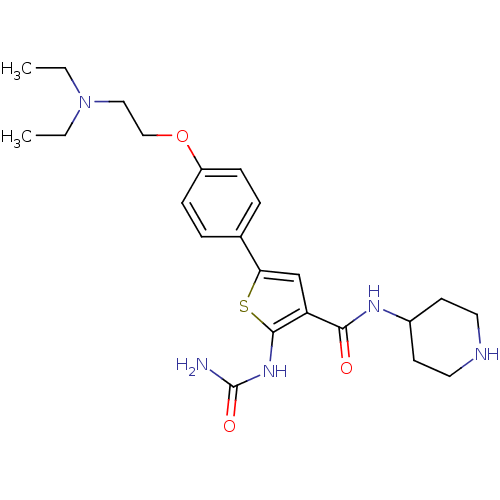
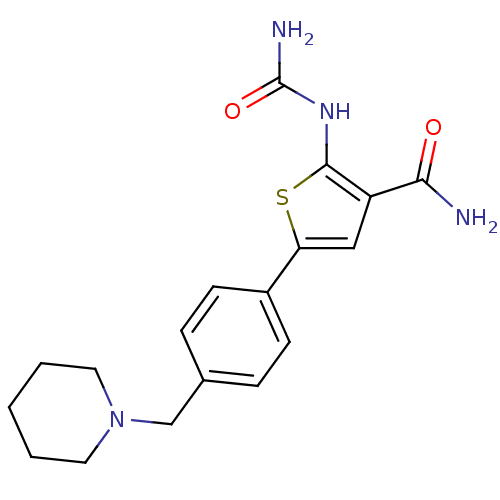
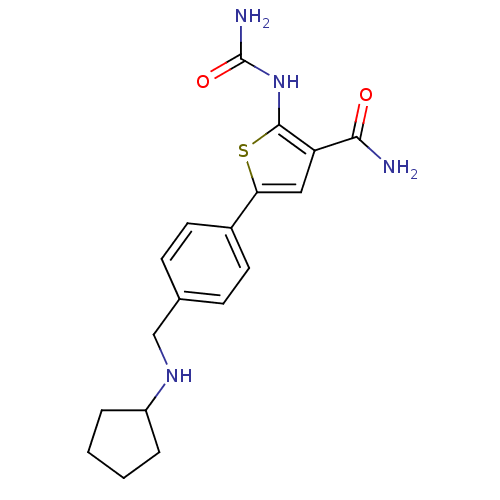
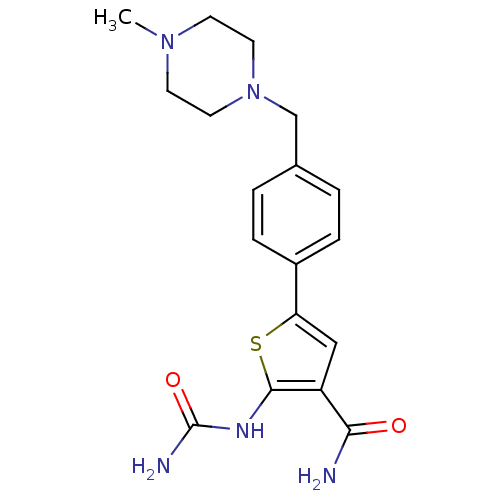
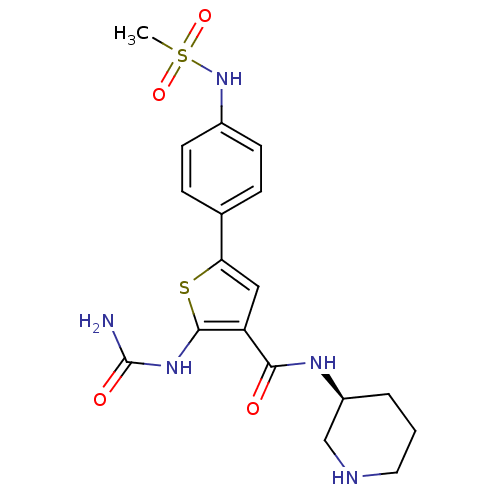
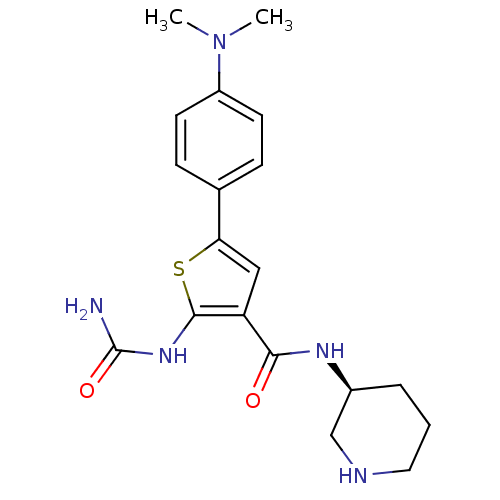
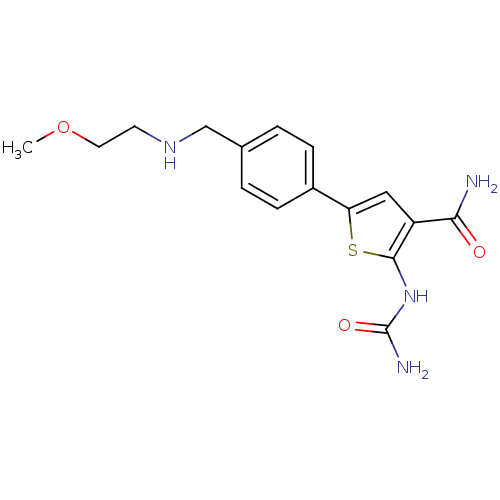
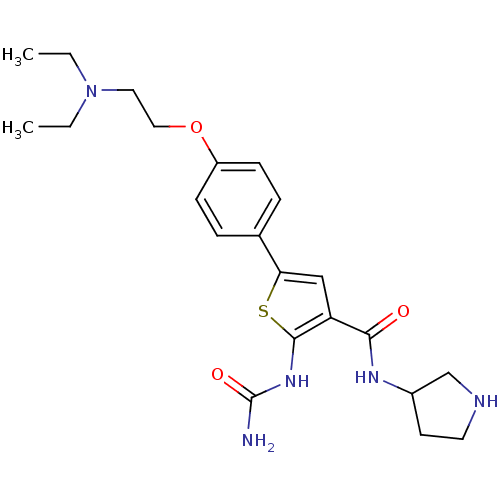
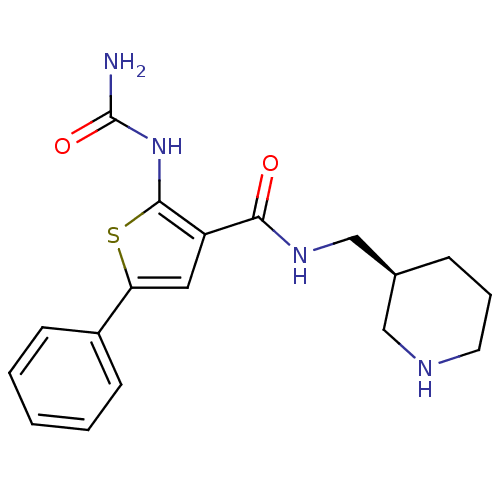
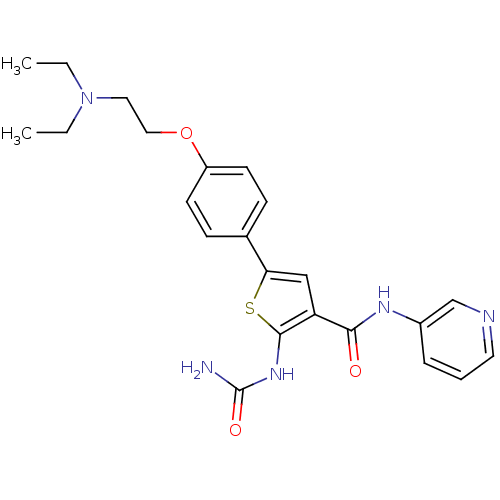
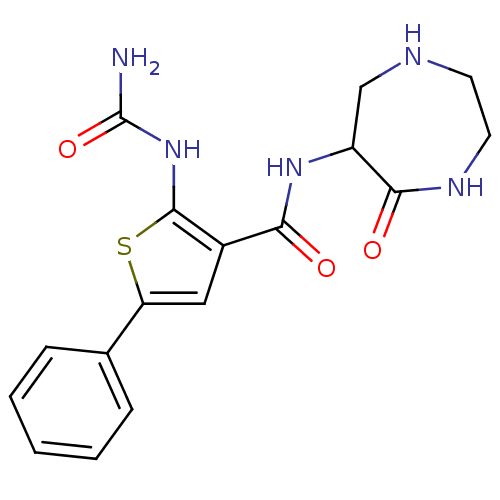
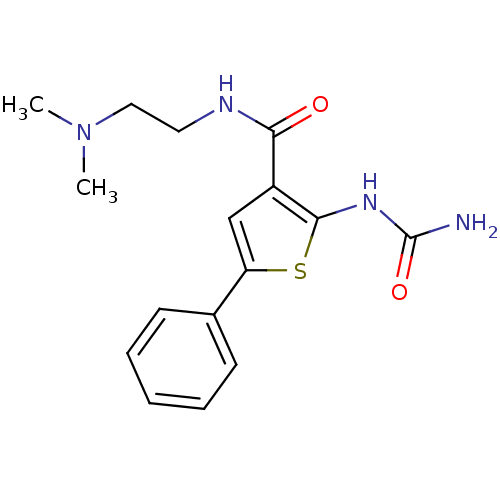
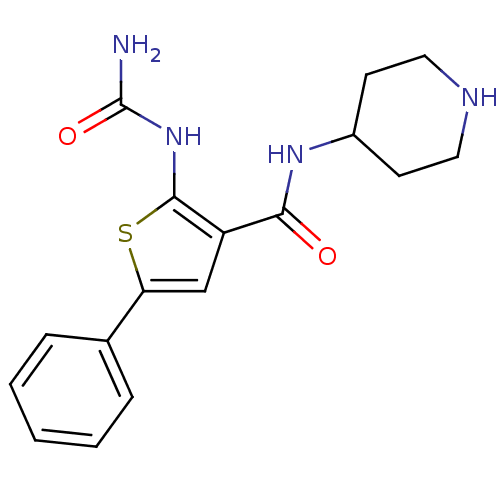
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL



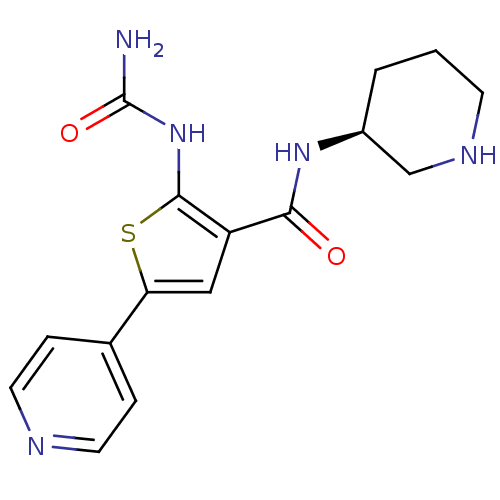


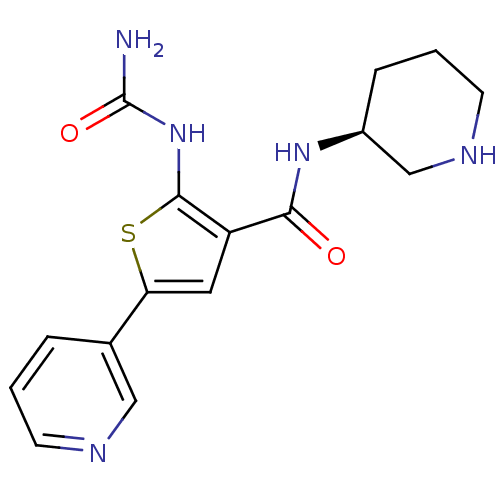
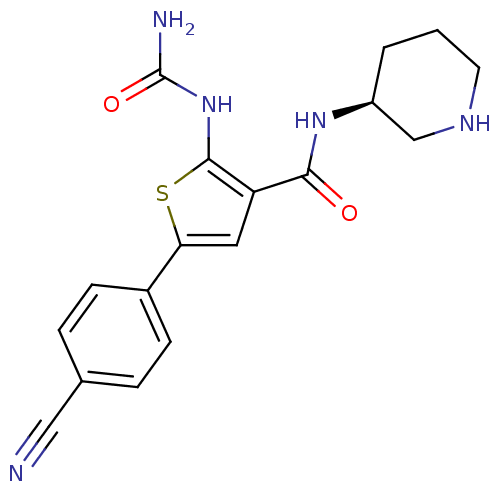


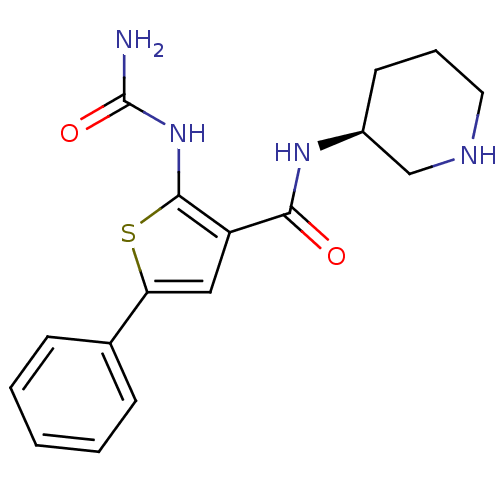
 3D Structure (crystal)
3D Structure (crystal)