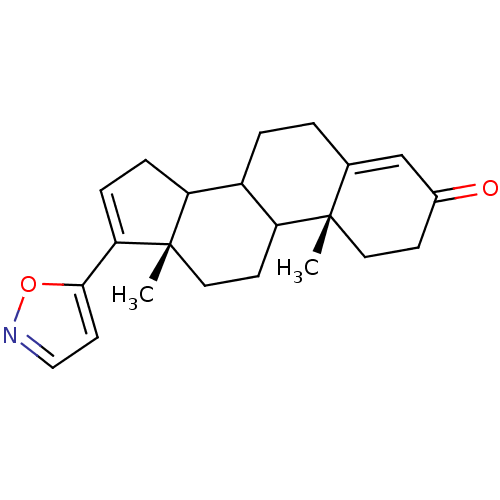
Affinity DataKi: 56.5nMAssay Description:In vitro inhibition of human Cytochrome P450 17A1 activityMore data for this Ligand-Target Pair
Affinity DataKi: 81.5nMAssay Description:In vitro inhibition of human Cytochrome P450 17A1 activityMore data for this Ligand-Target Pair
Affinity DataKi: 243nMAssay Description:In vitro inhibition of human Cytochrome P450 17A1 activityMore data for this Ligand-Target Pair
Affinity DataKi: 325nMAssay Description:In vitro inhibition of human Cytochrome P450 17A1 activityMore data for this Ligand-Target Pair
Affinity DataKi: 505nMAssay Description:In vitro inhibition of human Cytochrome P450 17A1 activityMore data for this Ligand-Target Pair
Affinity DataKi: 610nMAssay Description:In vitro inhibition of human Cytochrome P450 17A1 activityMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University of Maryland
Curated by ChEMBL
University of Maryland
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of [1-beta-2beta-3H]- -testosterone binding to human steroid 5-alpha-reductase type 2 of BPH tissue at 10 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Inhibition of ATRA-induced CYP26 in human T47D cell microsome assessed as ATRA metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:Inhibition of ATRA-induced CYP26 in human T47D cell microsome assessed as ATRA metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibition of ATRA-induced CYP26 in human T47D cells assessed as ATRA metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of ATRA-induced CYP26 in human T47D cells assessed as ATRA metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 10.9nMAssay Description:Inhibition of ATRA-induced CYP26 in human MCF7 cells assessed ATRA as metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of ATRA-induced CYP26 in human T47D cells assessed as ATRA metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 24.7nMAssay Description:Inhibition of ATRA-induced CYP26 in human MCF7 cells assessed ATRA as metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of ATRA-induced CYP26 in human T47D cell microsome assessed as ATRA metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:In vitro inhibition of human Cytochrome P450 17A1 activityMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibition of ATRA-induced CYP26 in human MCF7 cells assessed ATRA as metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University of Maryland
Curated by ChEMBL
University of Maryland
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of [1-beta-3H]-androstenedione binding to human steroid 5-alpha-reductase type I expressed in DU-145 cells at 10 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:In vitro inhibition of human Cytochrome P450 17A1 activityMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 178nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of ATRA-induced CYP26 in human MCF7 cells assessed ATRA as metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 215nMAssay Description:Inhibition of ATRA-induced CYP26 in human T47D cells assessed as ATRA metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:In vitro inhibition of human Cytochrome P450 17A1 activityMore data for this Ligand-Target Pair
Affinity DataIC50: 225nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 320nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 377nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 387nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 450nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University of Maryland
Curated by ChEMBL
University of Maryland
Curated by ChEMBL
Affinity DataIC50: 480nMAssay Description:Inhibition of [1-beta-2beta-3H]- -testosterone binding to human steroid 5-alpha-reductase type 2 of BPH tissue at 10 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: 562nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 617nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 628nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 660nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University of Maryland
Curated by ChEMBL
University of Maryland
Curated by ChEMBL
Affinity DataIC50: 770nMAssay Description:Inhibition of [1-beta-3H]-androstenedione binding to human steroid 5-alpha-reductase type I expressed in DU-145 cells at 10 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of ATRA-induced CYP26 in human T47D cell microsome assessed as ATRA metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of ATRA-induced CYP26 in human T47D cell microsome assessed as ATRA metabolism using [11.12-3H]-ATRAMore data for this Ligand-Target Pair
Affinity DataIC50: 915nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair
Affinity DataIC50: 915nMAssay Description:In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair

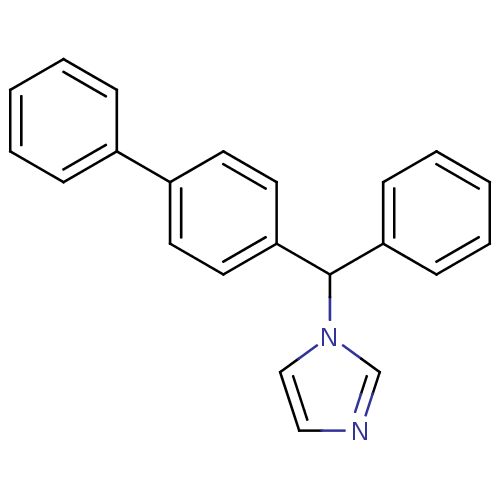
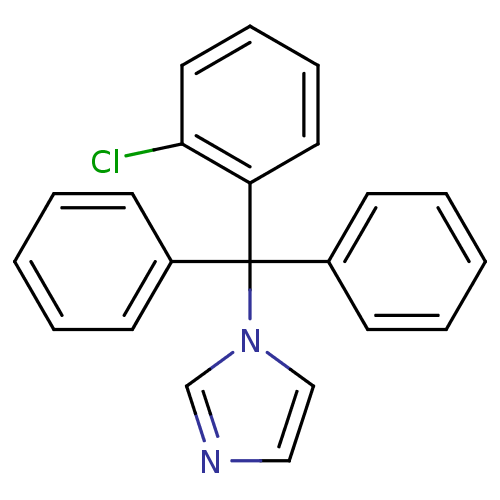
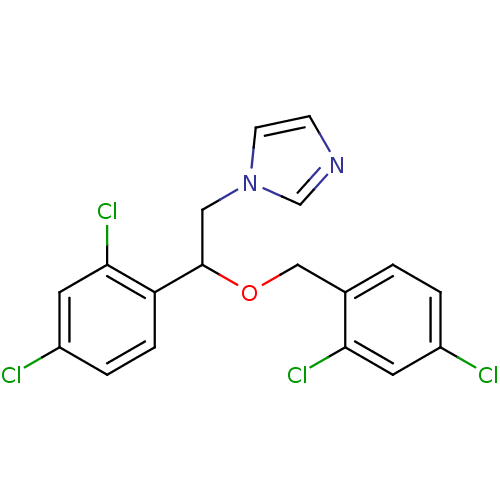
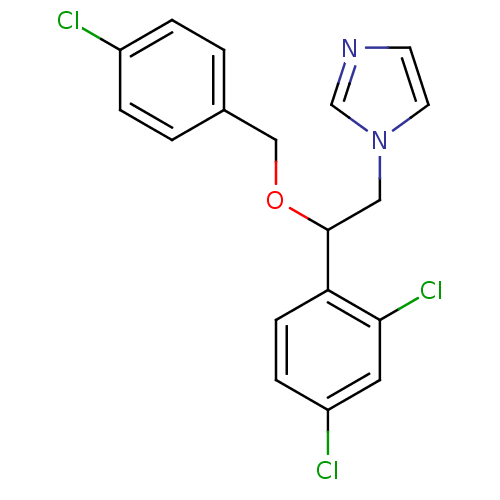
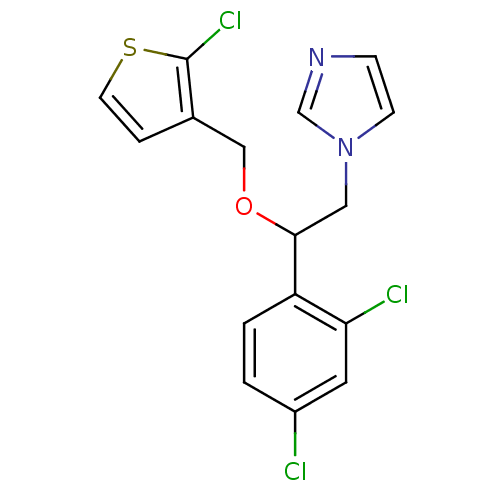
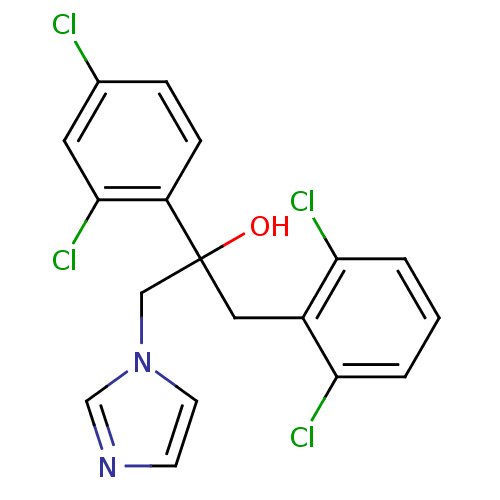

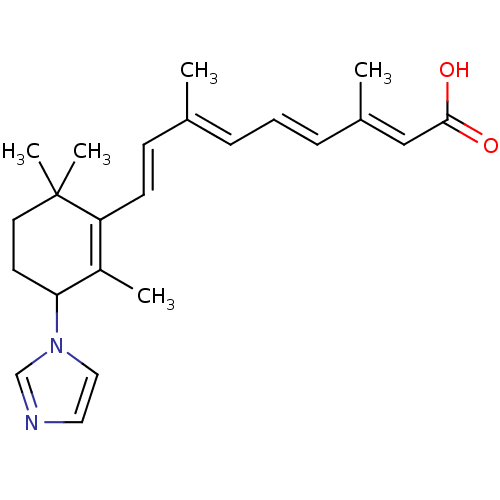
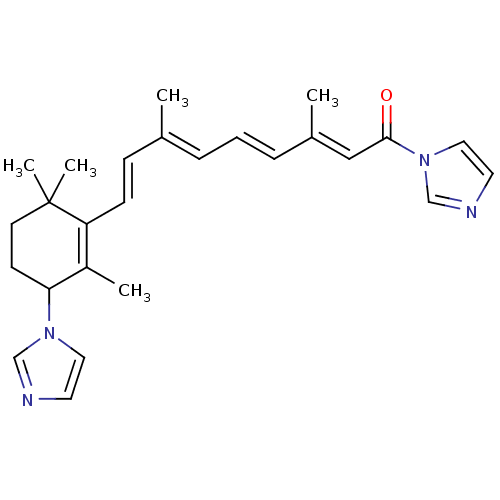
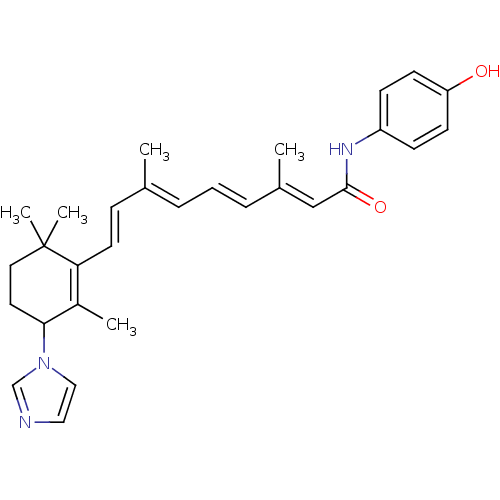
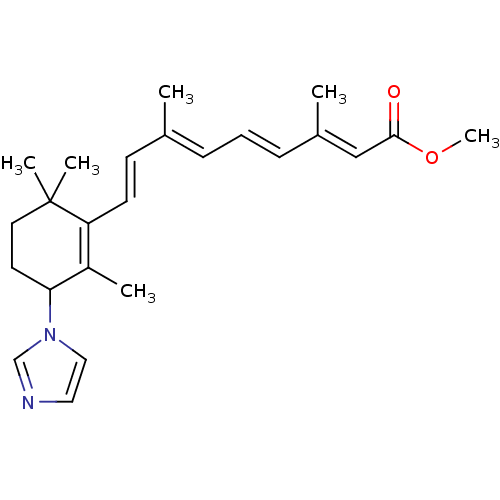
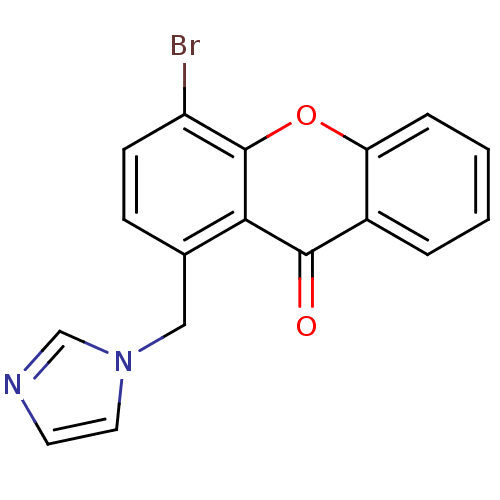

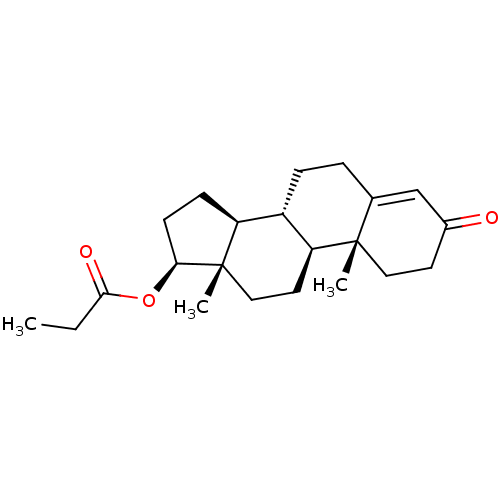

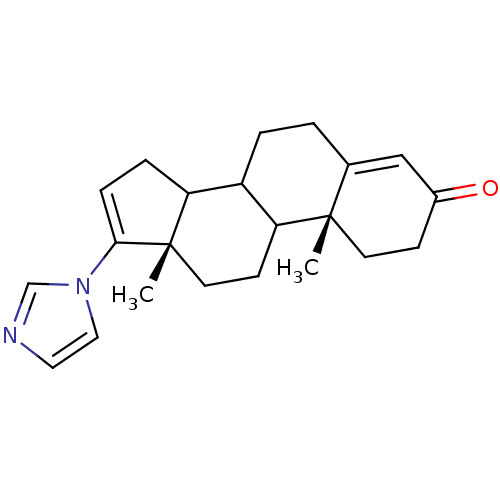
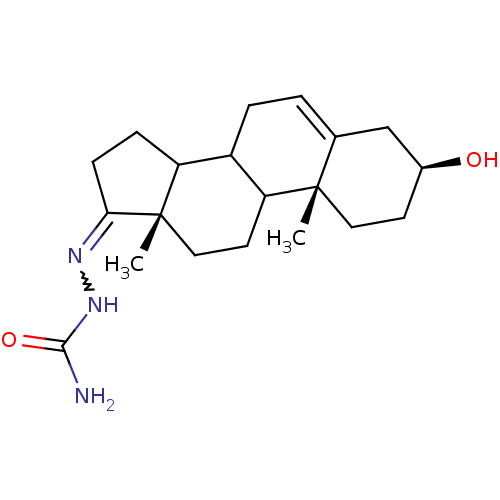


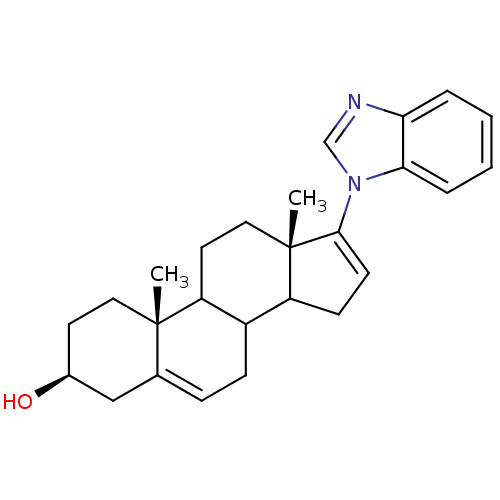


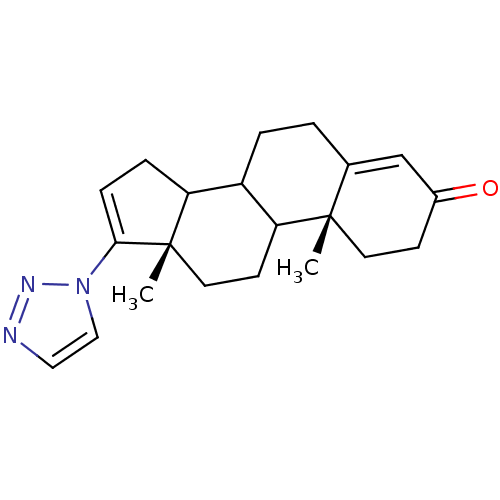
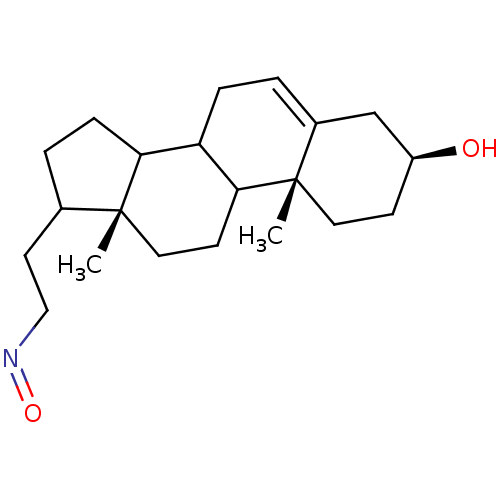
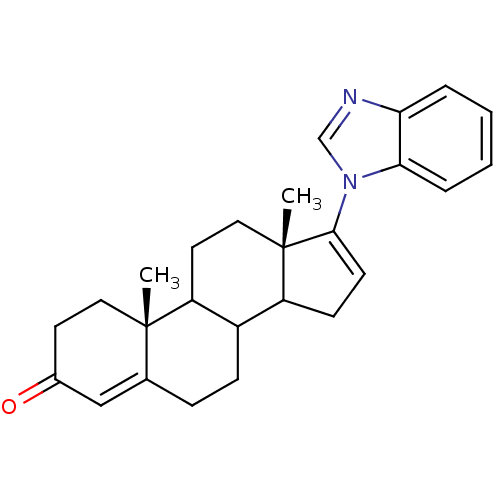
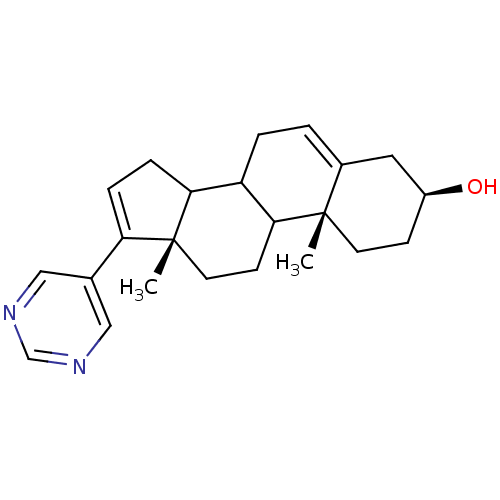
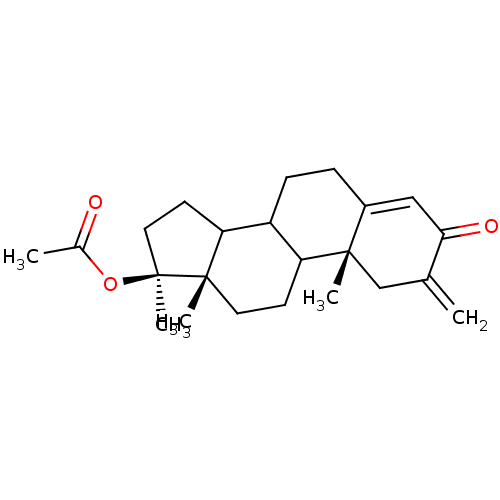

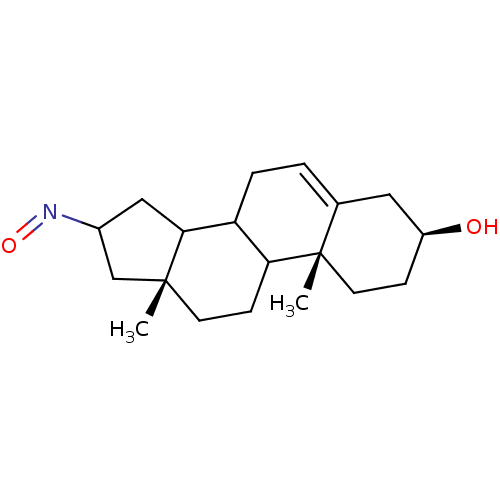
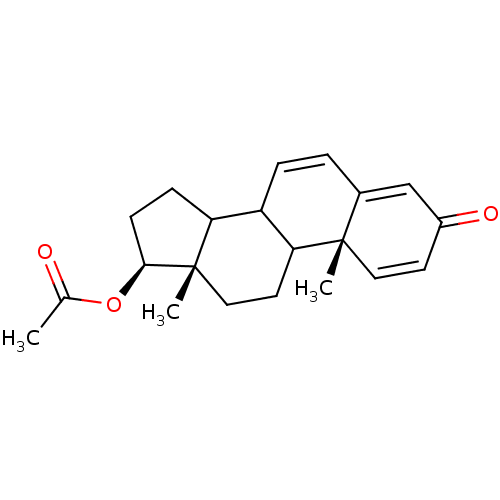


 3D Structure (crystal)
3D Structure (crystal)