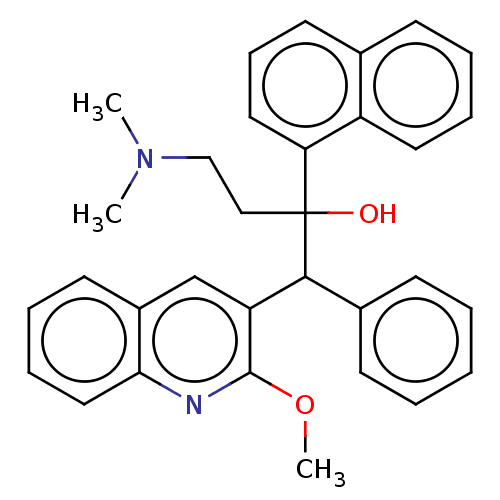
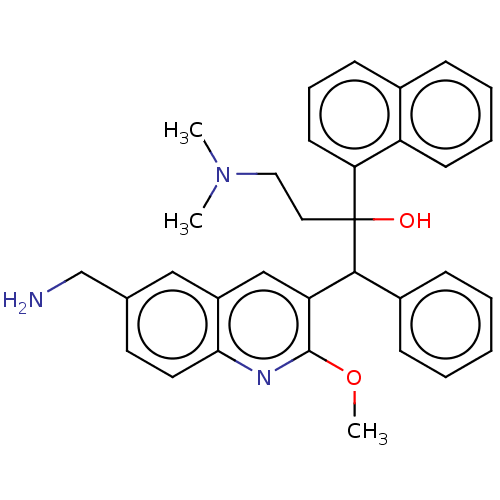
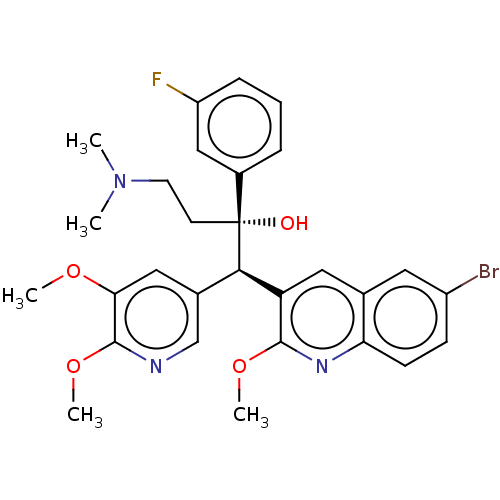
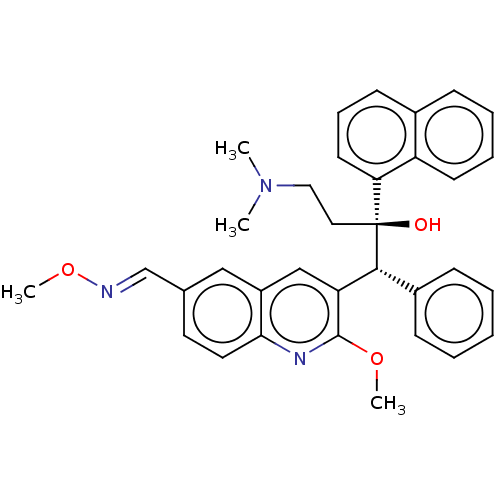
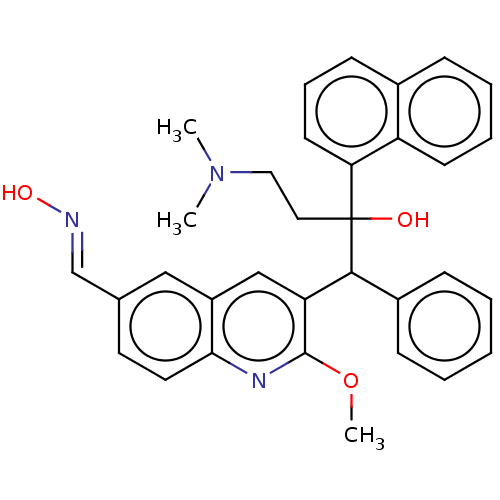
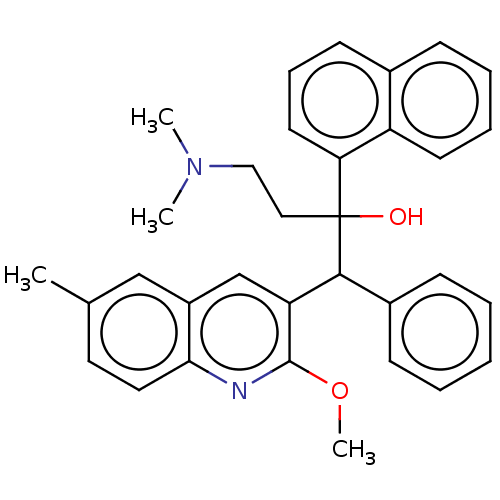
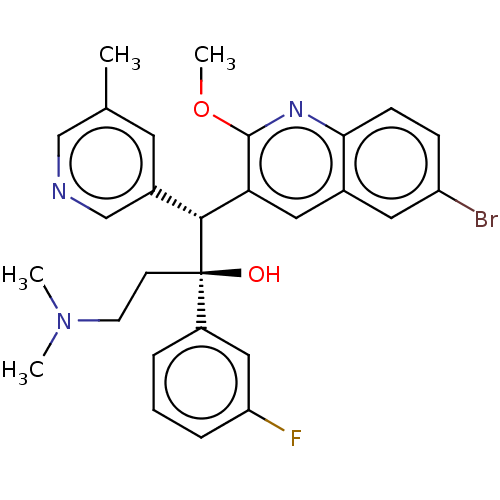
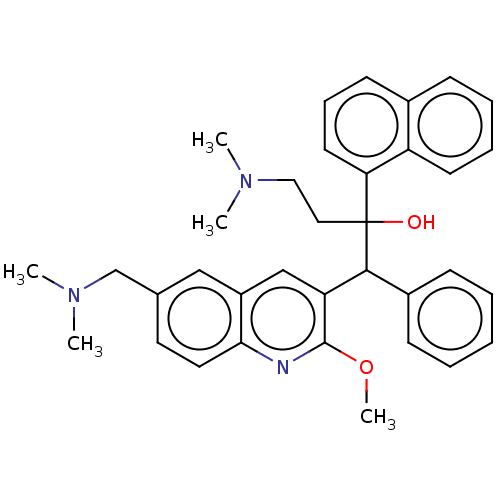
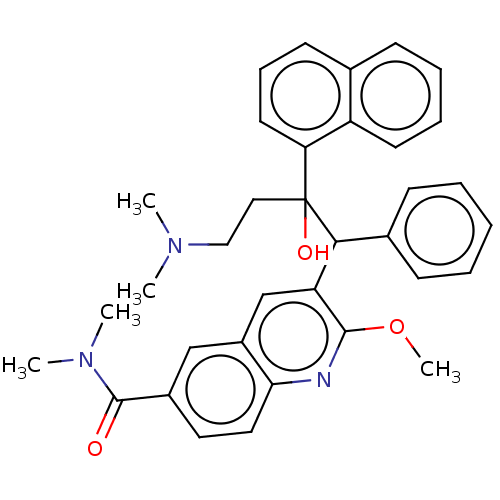
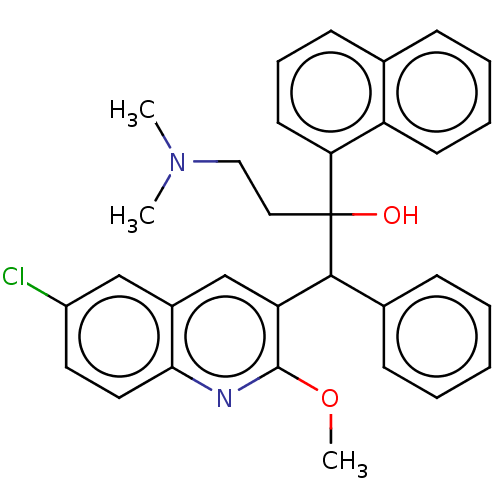
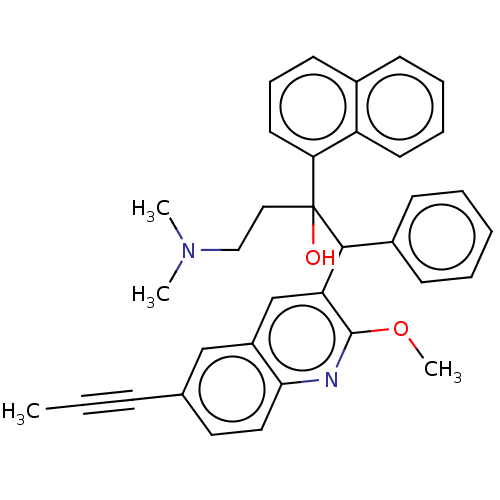
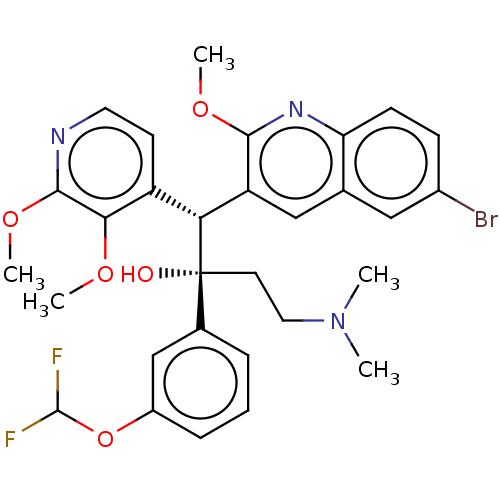
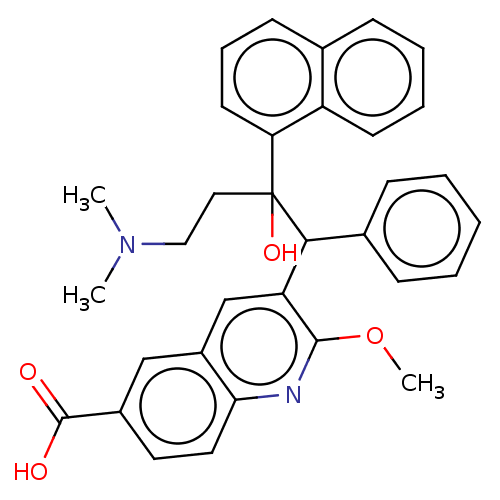
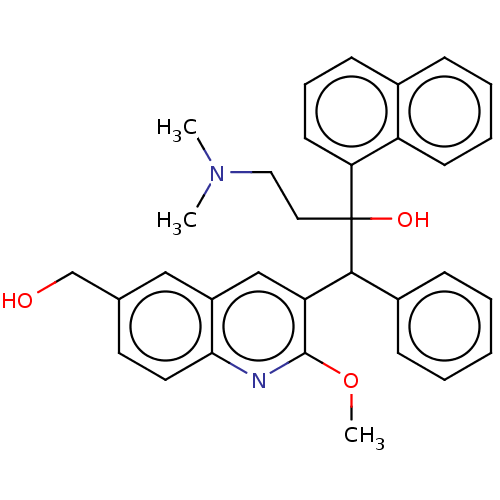
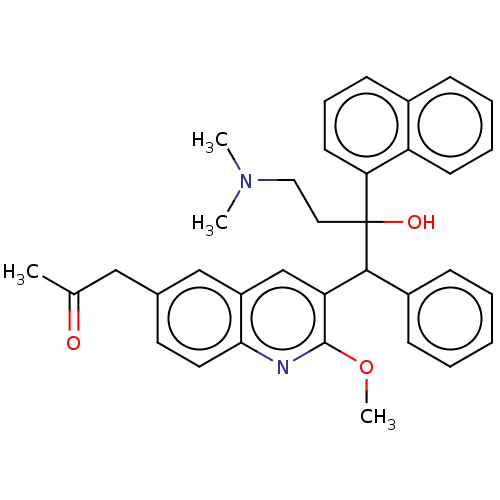
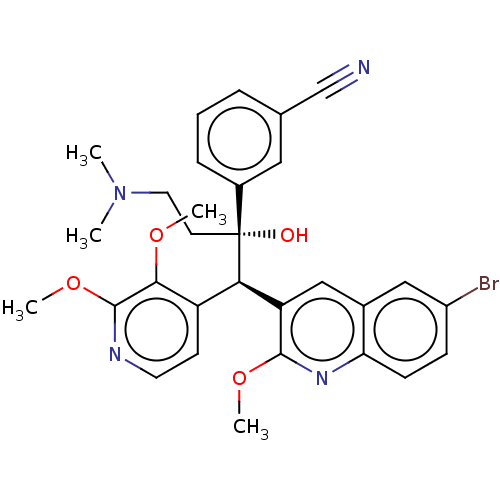
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 370nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 530nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 880nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 890nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Effective concentration on alkaline phosphatase activity in human T47D breast carcinoma cell line.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 4.60E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) after 20 minsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 6.30E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 6.90E+3nMAssay Description:Effective concentration on alkaline phosphatase activity in human T47D breast carcinoma cell line.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 7.90E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) after 20 minsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Effective concentration against PR (progesterone receptor)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) after 20 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) after 20 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) after 20 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) after 20 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) after 20 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) after 20 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) after 20 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) after 20 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DNA polymerase betaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DNA polymerase alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DNA polymerase betaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DNA polymerase gammaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DNA polymerase alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DNA polymerase gammaMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+7nMAssay Description:Inhibition of peripheral benzodiazepine BZD receptor by radioligand binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+7nMAssay Description:Inhibition of peripheral benzodiazepine BZD receptor by radioligand binding assayMore data for this Ligand-Target Pair