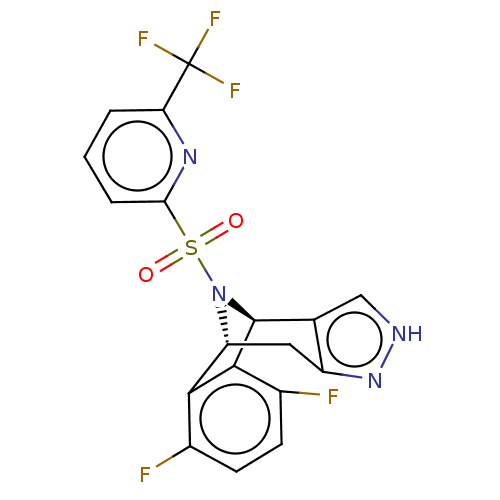
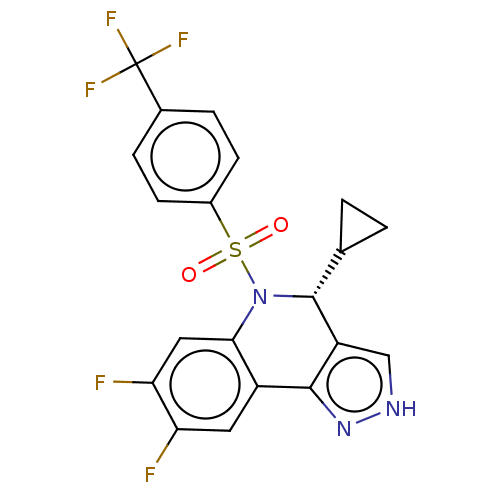
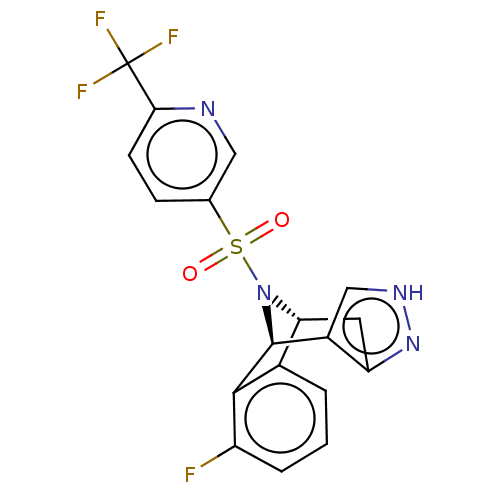
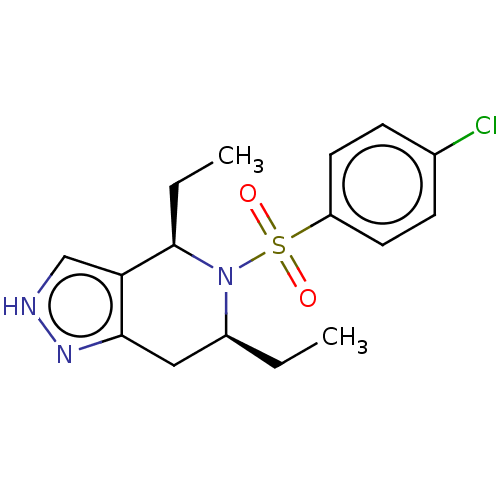
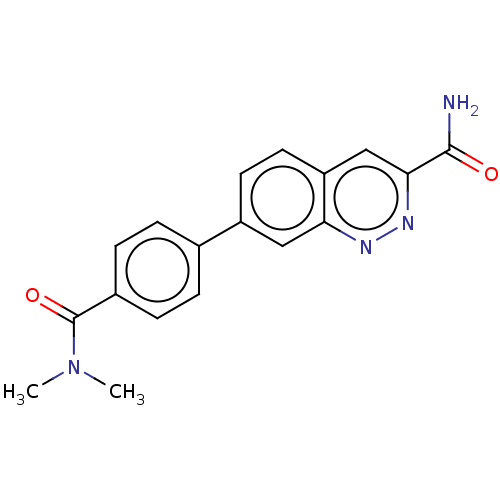
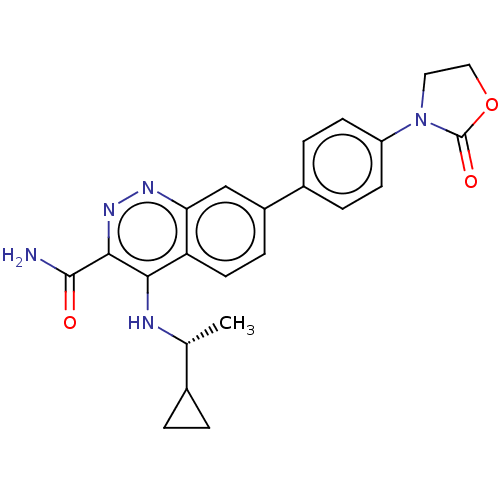
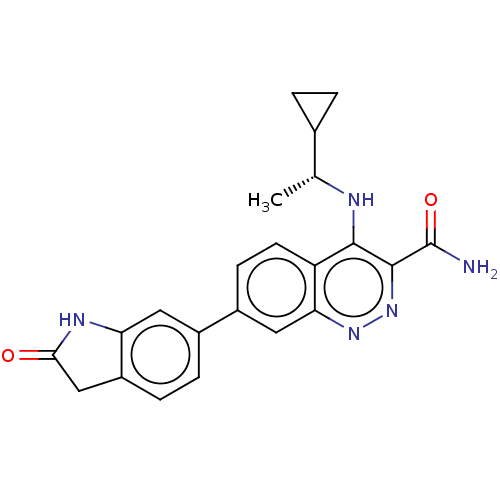
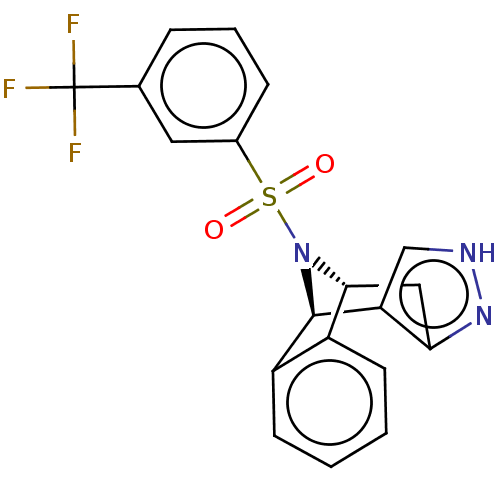
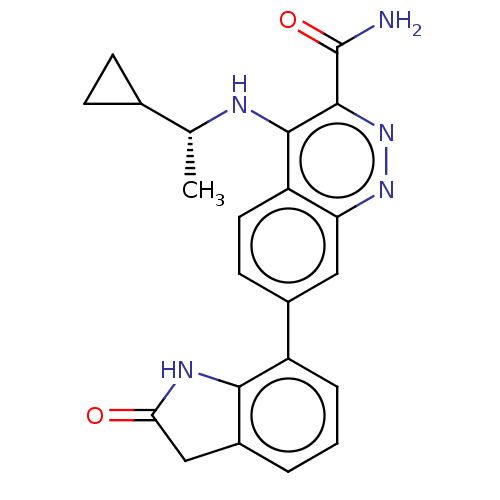
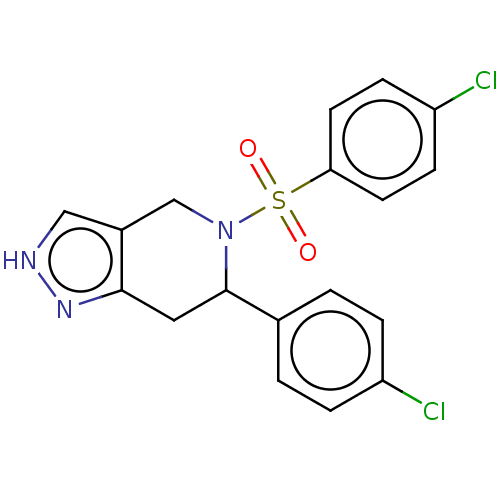
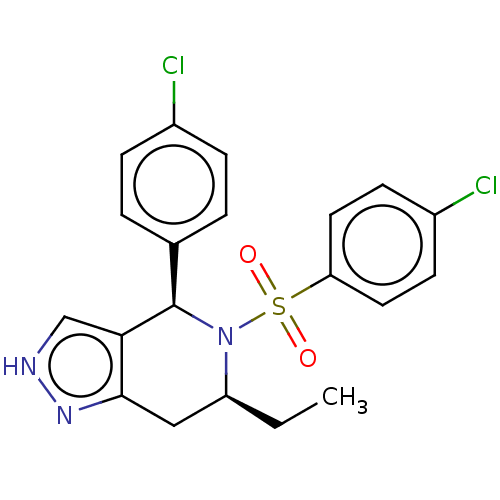
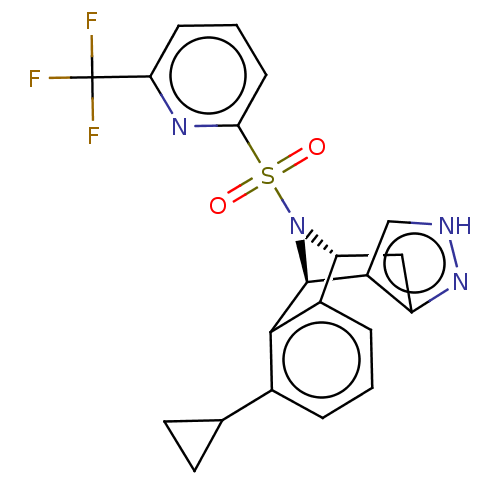
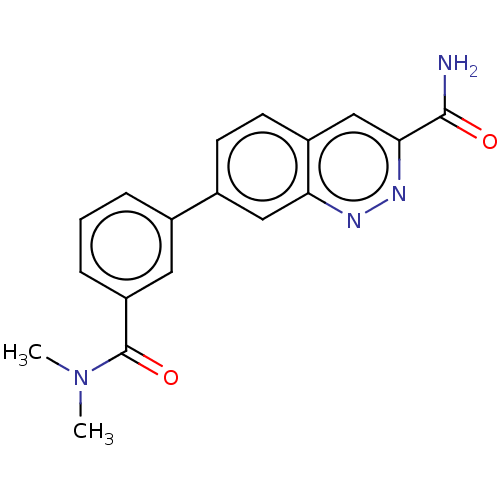
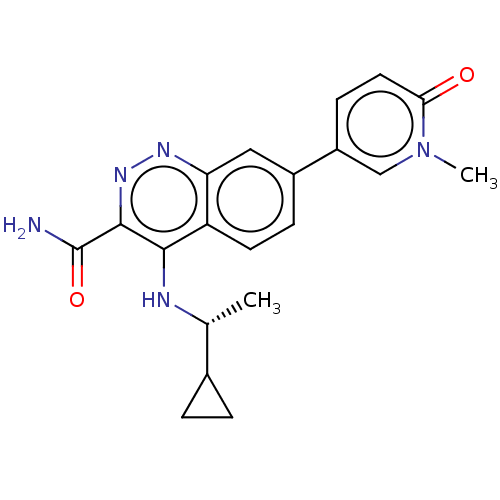
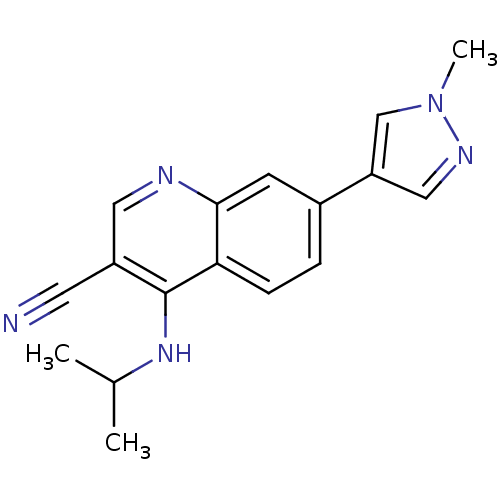
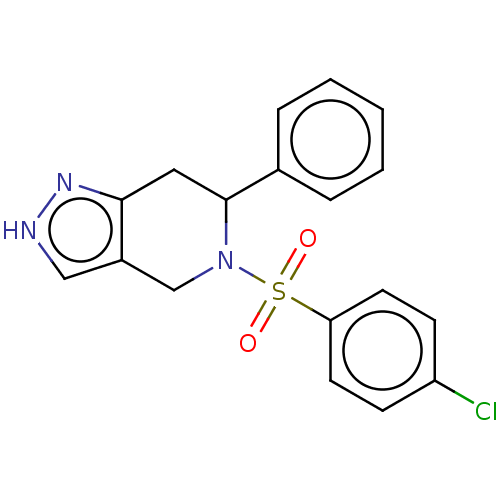
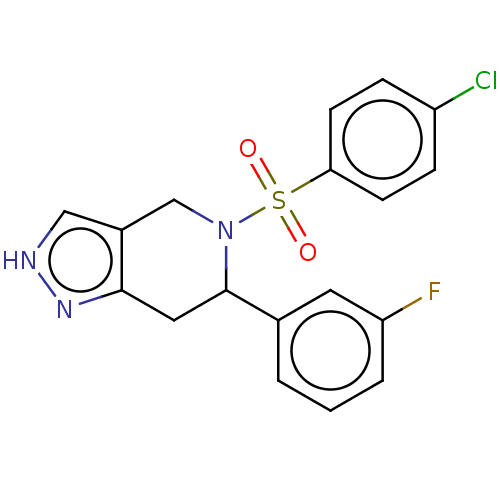
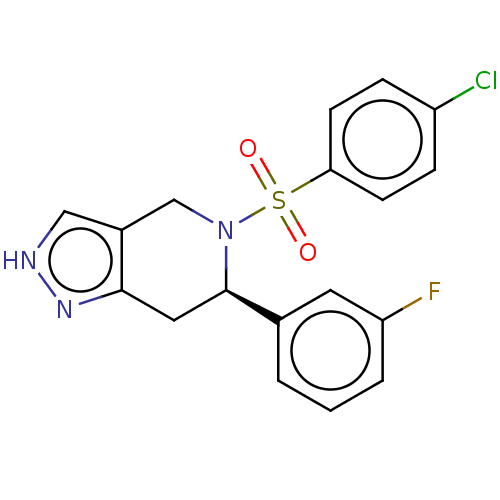
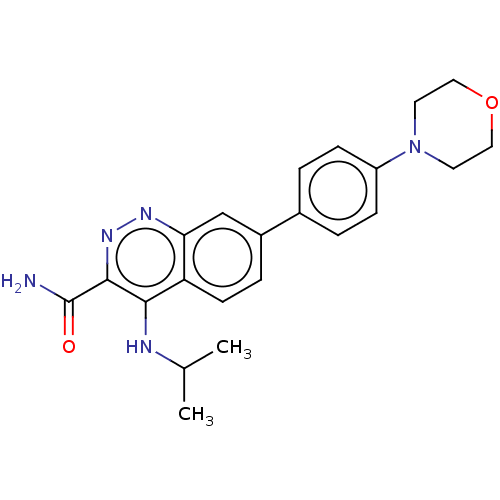
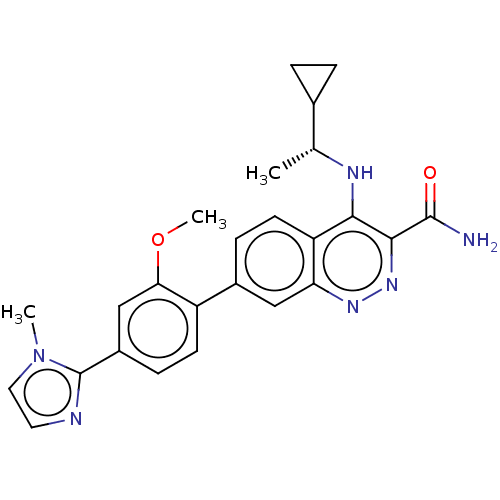
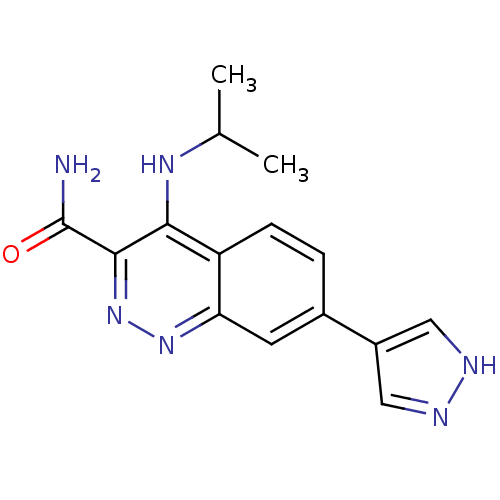
Affinity DataIC50: 0.130nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Inhibition of gamma-secretase-mediated APP cleavage in human IMR-32 cells assessed as amyloid beta40 level after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Inhibition of gamma-secretase-mediated APP cleavage in human IMR-32 cells assessed as amyloid beta40 level after 2 hrs by ELISAMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
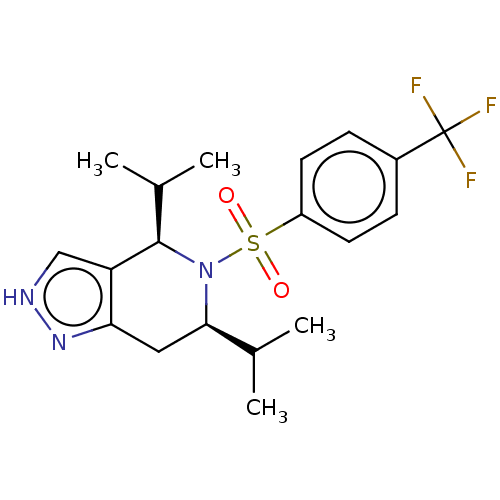
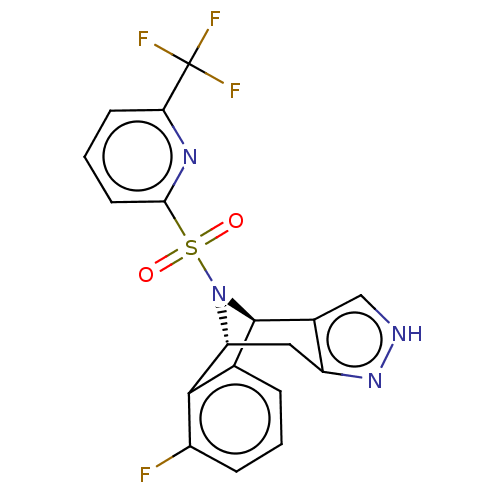
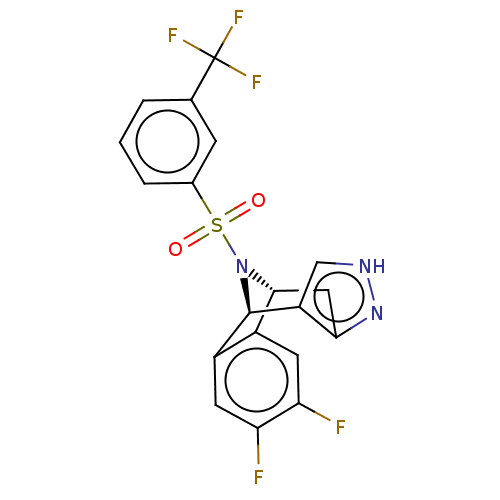
Affinity DataIC50: 0.5nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
Affinity DataIC50: 0.570nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:Inhibition of gamma-secretase-mediated APP cleavage in human IMR-32 cells assessed as amyloid beta40 level after 2 hrs by ELISAMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
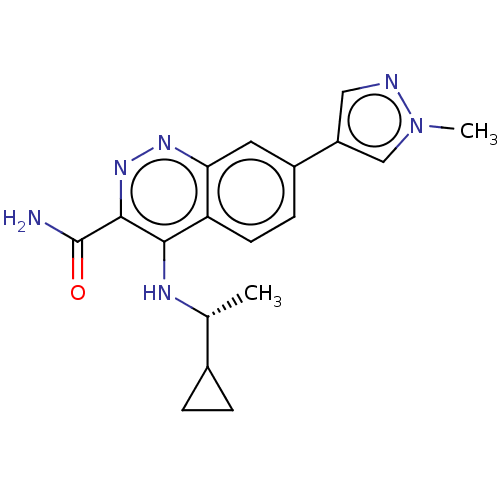
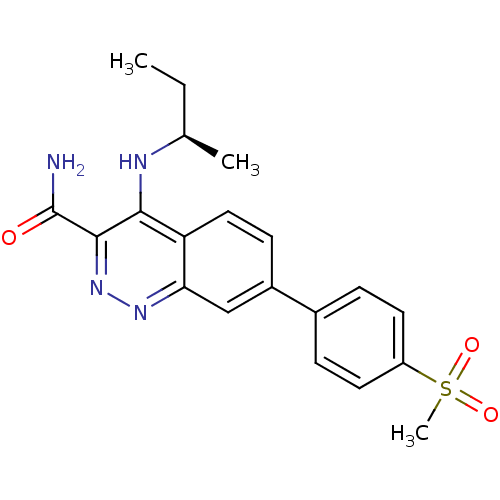
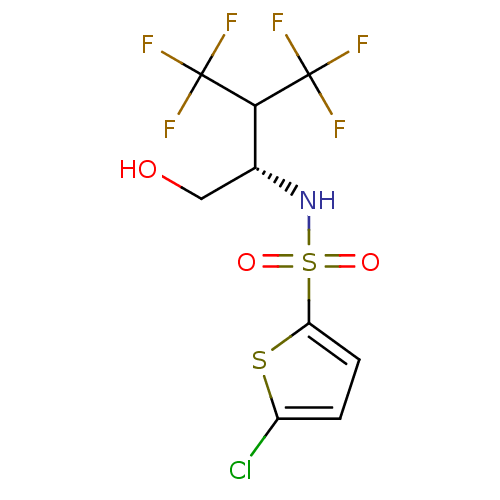
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
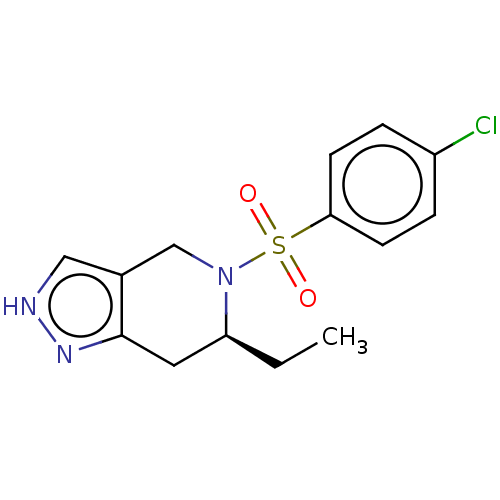
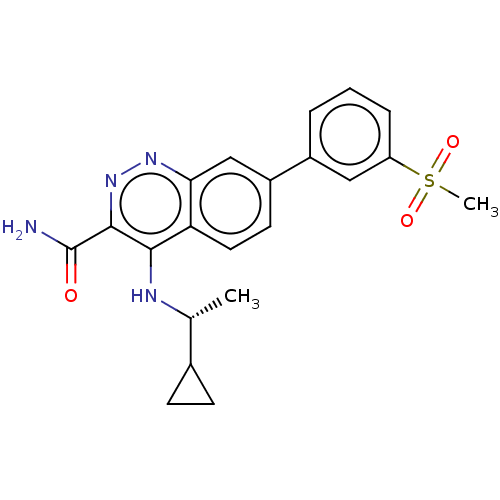
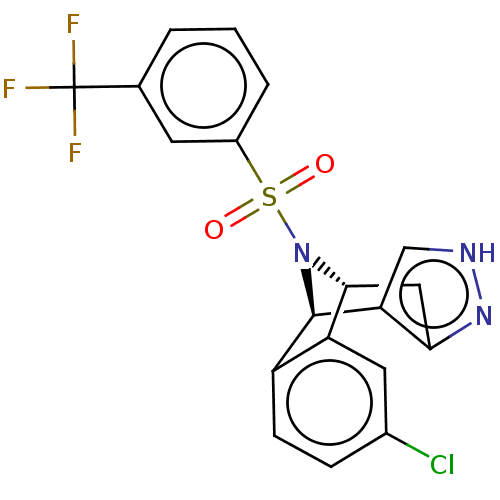
Affinity DataIC50: 1nMAssay Description:Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 1nMAssay Description:Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of gamma-secretase-mediated APP cleavage in human IMR-32 cells assessed as amyloid beta40 level after 2 hrs by ELISAMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 1nMAssay Description:Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using Notch as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 2nMAssay Description:Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibition of gamma-secretase-mediated APP cleavage in human IMR-32 cells assessed as amyloid beta40 level after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of gamma-secretase-mediated APP cleavage in human IMR-32 cells assessed as amyloid beta40 level after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of gamma-secretase-mediated notch cleavage in human IMR-32 cells after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of gamma-secretase-mediated notch cleavage in human IMR-32 cells after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using Notch as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:Inhibition of gamma-secretase-mediated APP cleavage in human IMR-32 cells assessed as amyloid beta40 level after 2 hrs by ELISAMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 7nMAssay Description:Inhibition of wild type GST-tagged LRRK2 (970-2527 amino acids)(unknown origin) assessed as inhibition of LRRKtide phosphorylation by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.70nMAssay Description:Inhibition of gamma-secretase-mediated APP cleavage in human IMR-32 cells assessed as amyloid beta40 level after 2 hrs by ELISAMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 8nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
Affinity DataIC50: 8.30nMAssay Description:Inhibition of gamma-secretase-mediated APP cleavage in human IMR-32 cells assessed as amyloid beta40 level after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 8.30nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 9nMAssay Description:Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 9nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 9nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 9nMAssay Description:Inhibition of wild type GST-tagged LRRK2 (970-2527 amino acids)(unknown origin) assessed as inhibition of LRRKtide phosphorylation by HTRF assayMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 9nMpH: 7.5 T: 2°CAssay Description:Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Imago Pharmaceuticals, Inc.
US Patent
Imago Pharmaceuticals, Inc.
US Patent
Affinity DataIC50: 9nMAssay Description:Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)