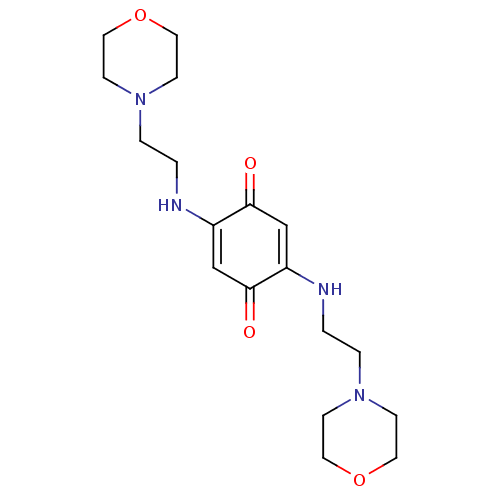
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Liaoning Normal University
Curated by ChEMBL
Liaoning Normal University
Curated by ChEMBL
Affinity DataIC50: 2.73E+4nMAssay Description:Inhibition of Helicobacter pylori urease assessed as ammonia production preincubated for 3 hrs measured by indophenol methodMore data for this Ligand-Target Pair
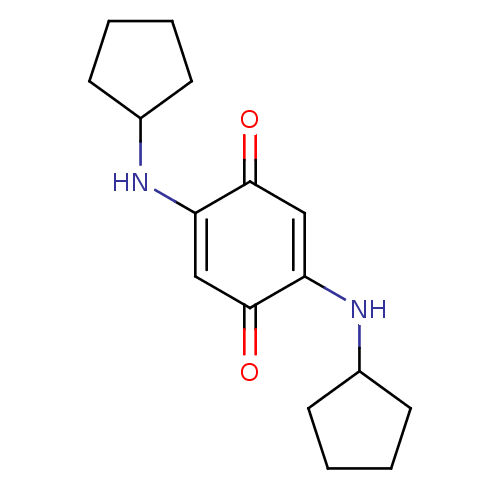
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Liaoning Normal University
Curated by ChEMBL
Liaoning Normal University
Curated by ChEMBL
Affinity DataIC50: 3.54E+4nMAssay Description:Inhibition of Helicobacter pylori urease assessed as ammonia production preincubated for 3 hrs measured by indophenol methodMore data for this Ligand-Target Pair
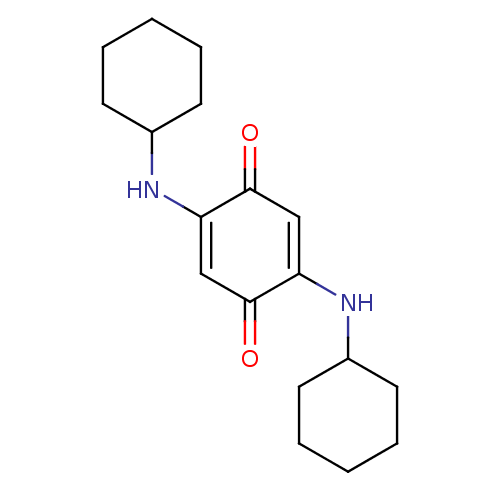
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Liaoning Normal University
Curated by ChEMBL
Liaoning Normal University
Curated by ChEMBL
Affinity DataIC50: 4.15E+4nMAssay Description:Inhibition of Helicobacter pylori urease assessed as ammonia production preincubated for 3 hrs measured by indophenol methodMore data for this Ligand-Target Pair
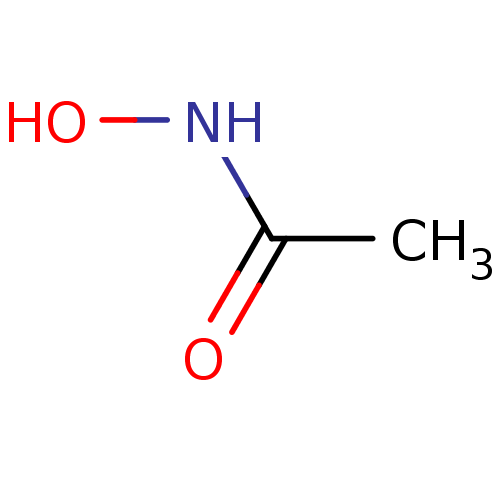
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Liaoning Normal University
Curated by ChEMBL
Liaoning Normal University
Curated by ChEMBL
Affinity DataIC50: 4.63E+4nMAssay Description:Inhibition of Helicobacter pylori urease assessed as ammonia production preincubated for 3 hrs measured by indophenol methodMore data for this Ligand-Target Pair
Affinity DataKd: 2.00E+3nMAssay Description:Binding affinity to human DDR1More data for this Ligand-Target Pair
Affinity DataKd: 31nMAssay Description:Binding affinity to human FLT1More data for this Ligand-Target Pair
Affinity DataKd: 62nMAssay Description:Binding affinity to human PDGFRAMore data for this Ligand-Target Pair
Affinity DataKd: 62nMAssay Description:Binding affinity to human RETMore data for this Ligand-Target Pair
Affinity DataKd: 10nMAssay Description:Binding affinity to human ABL2More data for this Ligand-Target Pair
Affinity DataKd: >1.00E+4nMAssay Description:Binding affinity to human FLT3More data for this Ligand-Target Pair
Affinity DataKd: >1.00E+4nMAssay Description:Binding affinity to human FLT4More data for this Ligand-Target Pair
Affinity DataKd: 28nMAssay Description:Binding affinity to human CSF1RMore data for this Ligand-Target Pair
Affinity DataKd: 0.580nMAssay Description:Binding affinity to human CSF1RMore data for this Ligand-Target Pair
Affinity DataKd: 337nMAssay Description:Binding affinity to human recombinant Bcl-2 expressed in Escherichia coli by Surface plasmon resonance assayMore data for this Ligand-Target Pair
Affinity DataKd: 100nMAssay Description:Binding affinity to human NTRK1More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)



