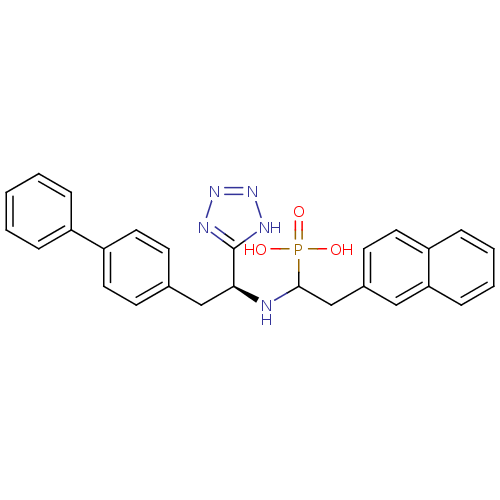
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 6.90nMAssay Description:Inhibitory concentration against human ECE-1 by RIAMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 10.4nMAssay Description:Inhibitory concentration against human ECE-1 by RIAMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibitory concentration against human ECE-1 by RIAMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibitory concentration against human ECE-1 by RIAMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 49.7nMAssay Description:Inhibitory concentration against human ECE-1 by RIAMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 61.3nMAssay Description:Inhibitory concentration against human ECE-1 by RIAMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 67nMAssay Description:Inhibitory concentration against human ECE-1 expressed in MDCK cellsMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 72.9nMAssay Description:Inhibitory concentration against human ECE-1 expressed in MDCK cellsMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 76.5nMAssay Description:Inhibitory concentration against human ECE-1 expressed in MDCK cellsMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 80.8nMAssay Description:Inhibitory concentration against human ECE-1 by RIAMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 481nMAssay Description:Inhibitory concentration against human ECE-1 expressed in MDCK cellsMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibitory concentration against human ECE-1 by RIAMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 1.09E+3nMAssay Description:Inhibitory concentration against human ECE-1 expressed in MDCK cellsMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 2.53E+3nMAssay Description:Inhibitory concentration against human ECE-1 expressed in MDCK cellsMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 1.09E+4nMAssay Description:Inhibitory concentration against human ECE-1 expressed in MDCK cellsMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 1.34E+4nMAssay Description:Inhibitory concentration against human angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 1.46E+4nMAssay Description:Inhibitory concentration against human angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetEndothelin-converting enzyme 1(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory concentration against human ECE-1 by RIAMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 3.15E+4nMAssay Description:Inhibitory concentration against human angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 3.66E+4nMAssay Description:Inhibitory concentration against human angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 3.92E+4nMAssay Description:Inhibitory concentration against NeprilysinMore data for this Ligand-Target Pair
Affinity DataIC50: 8.19E+4nMAssay Description:Inhibitory concentration against NeprilysinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against NeprilysinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against NeprilysinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against NeprilysinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against NeprilysinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against NeprilysinMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against human angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against human angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
University Institute of Pathology
Curated by ChEMBL
University Institute of Pathology
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against human angiotensin I converting enzymeMore data for this Ligand-Target Pair

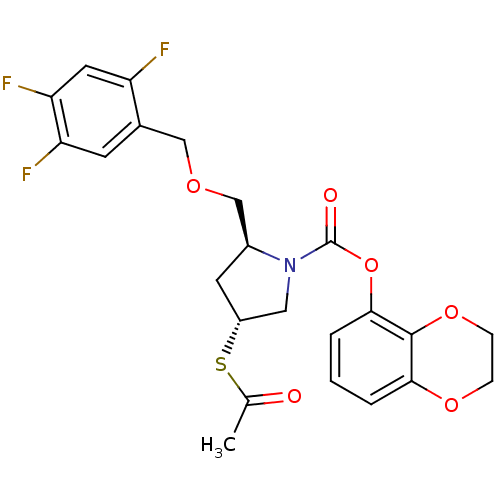
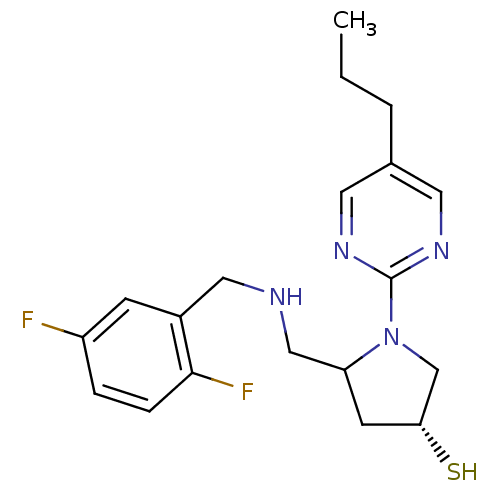
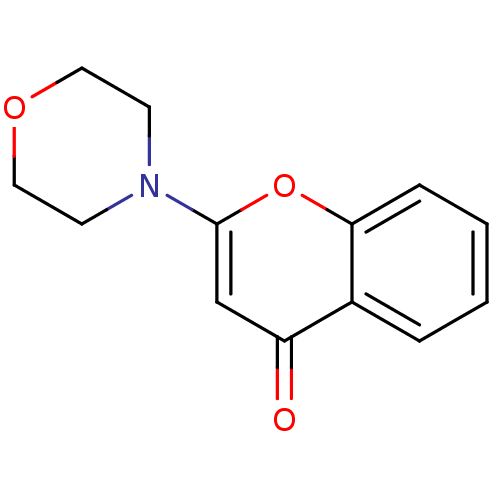
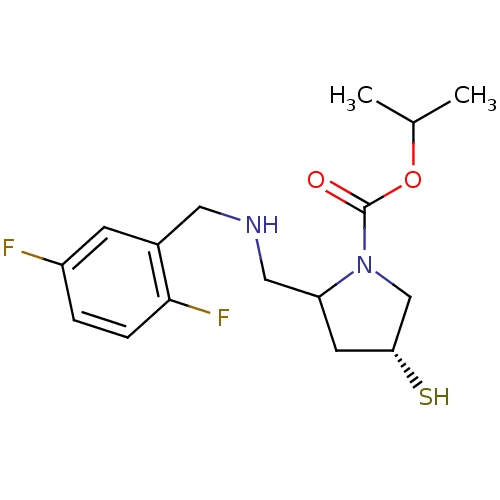
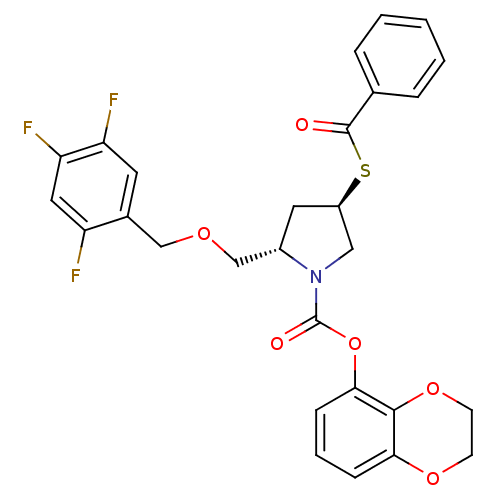
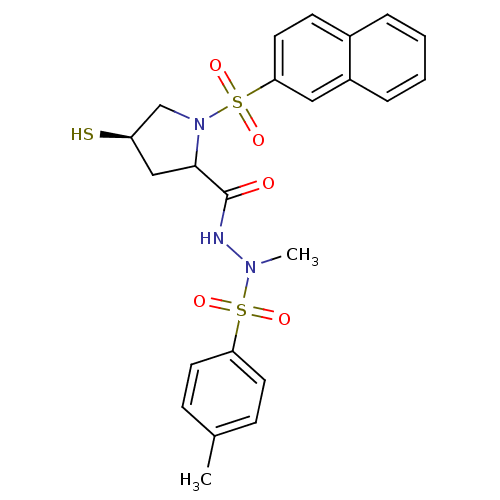
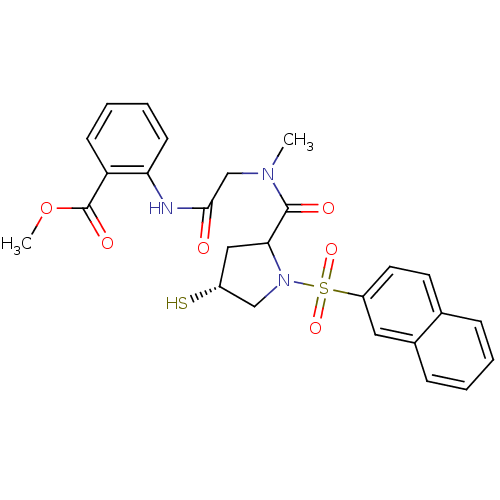

 3D Structure (crystal)
3D Structure (crystal)