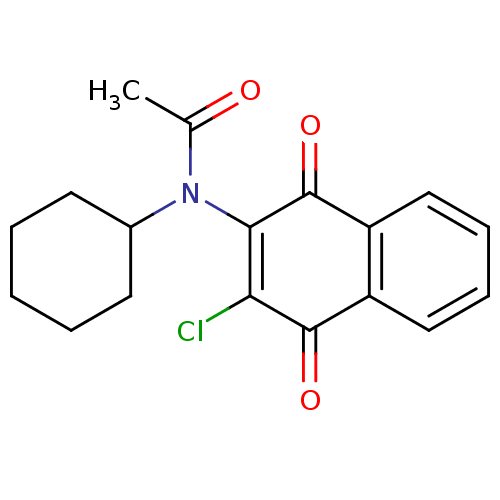
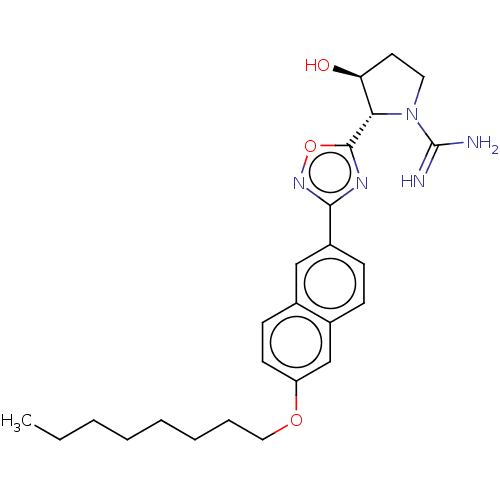
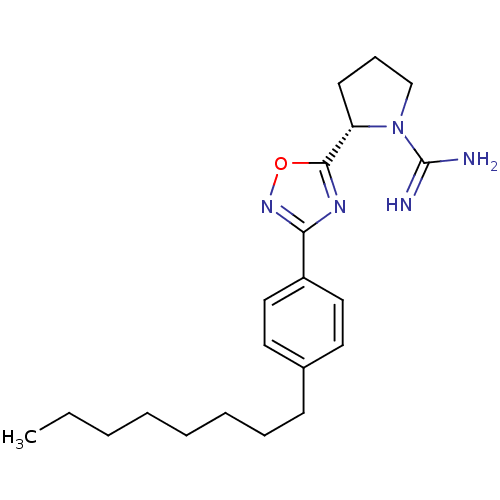
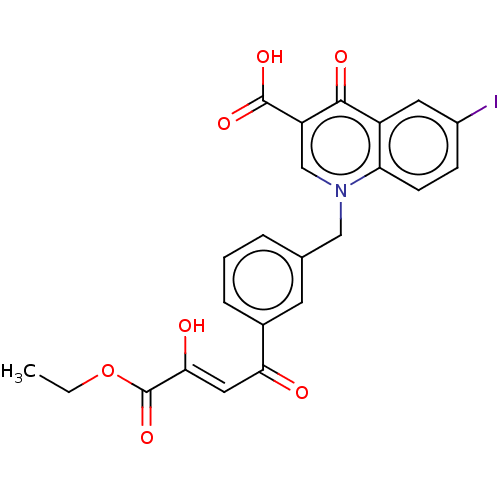
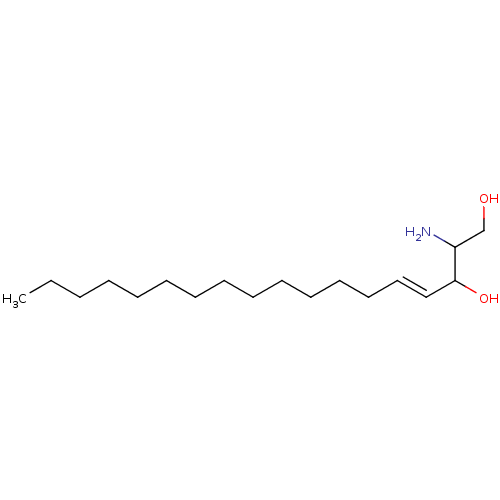
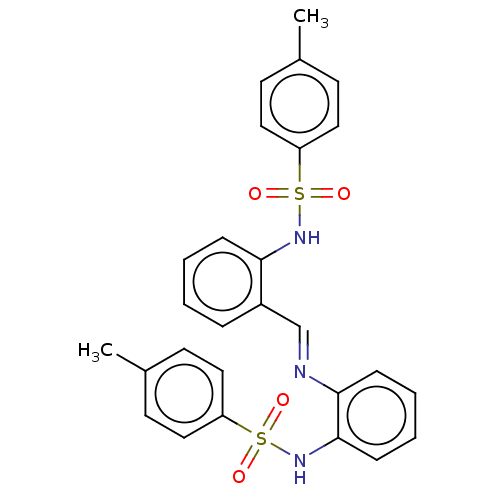
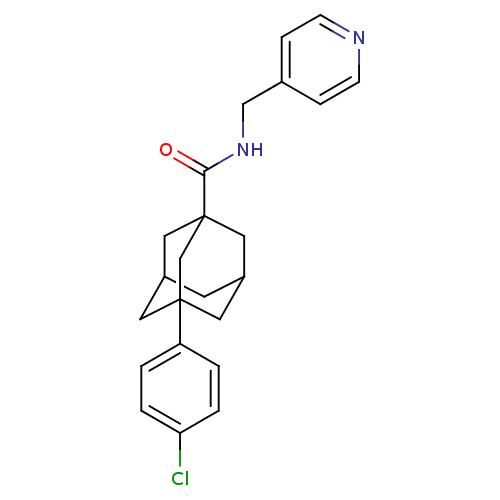
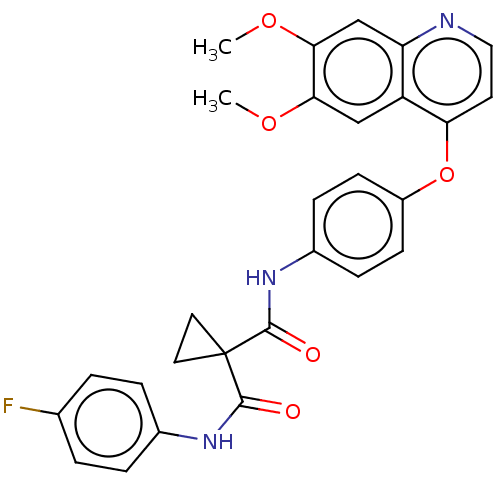
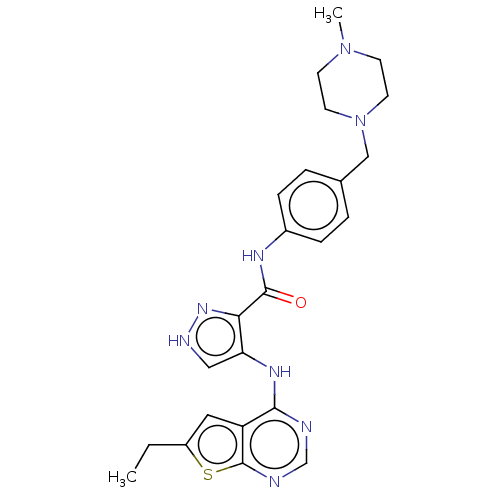
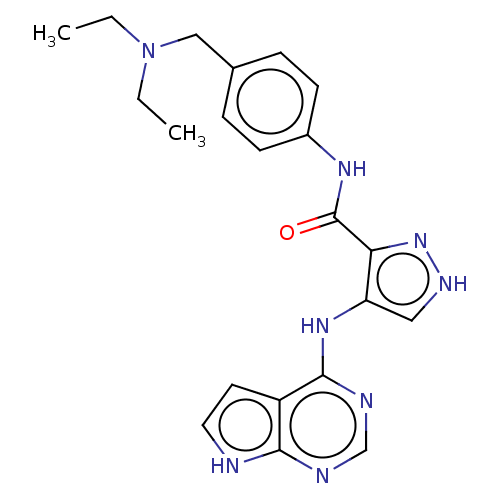
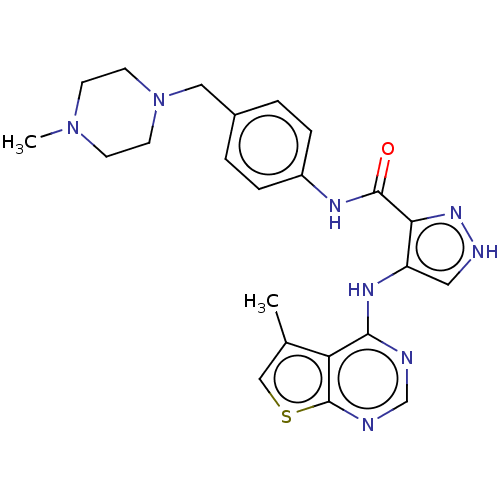
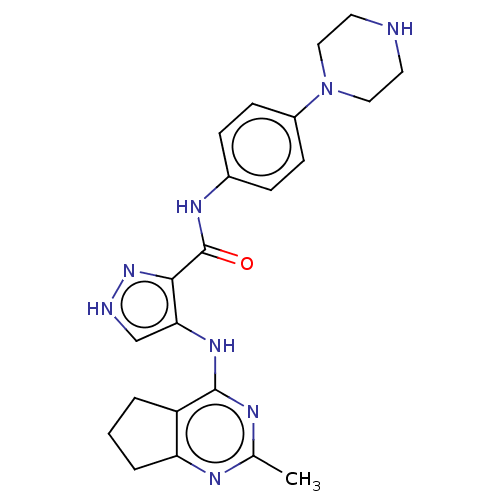
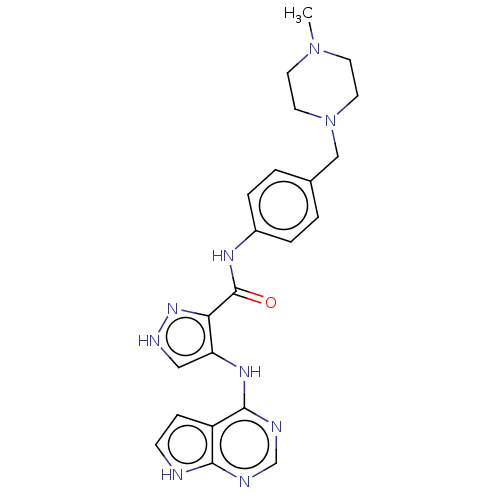
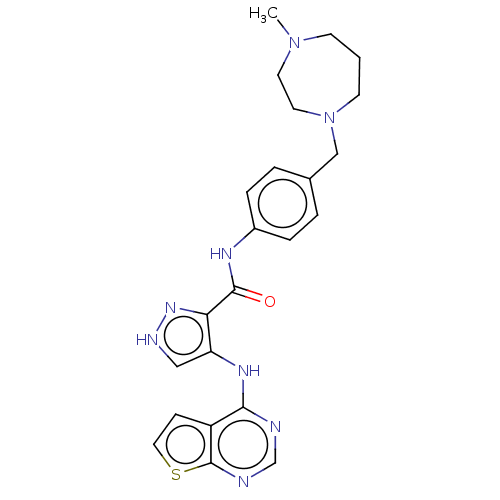
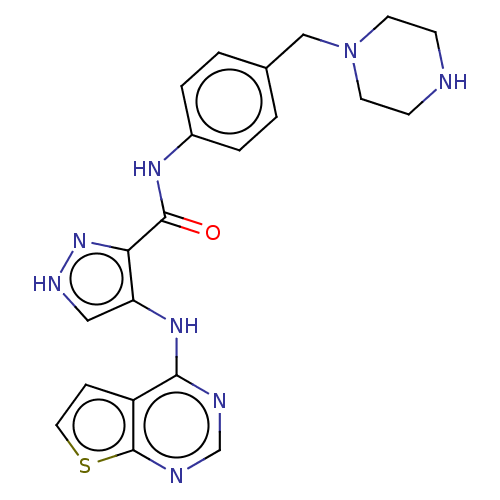
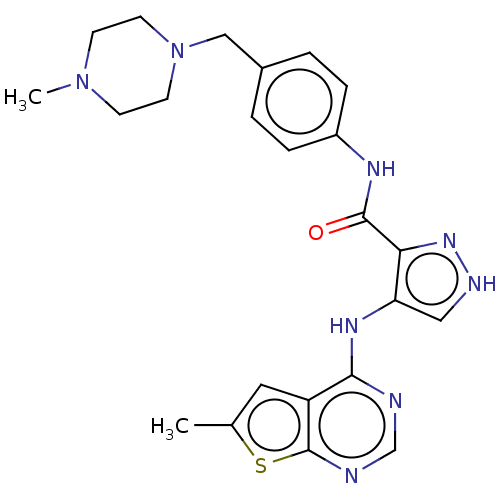
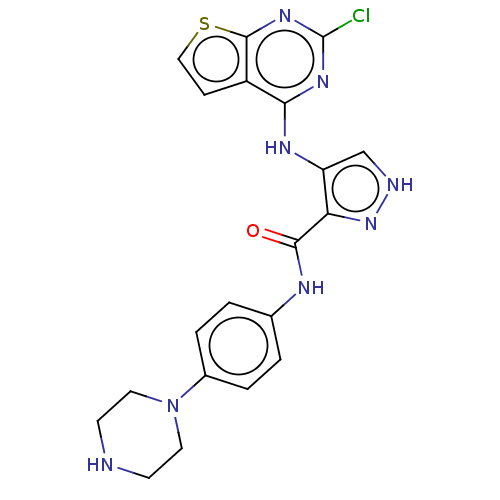
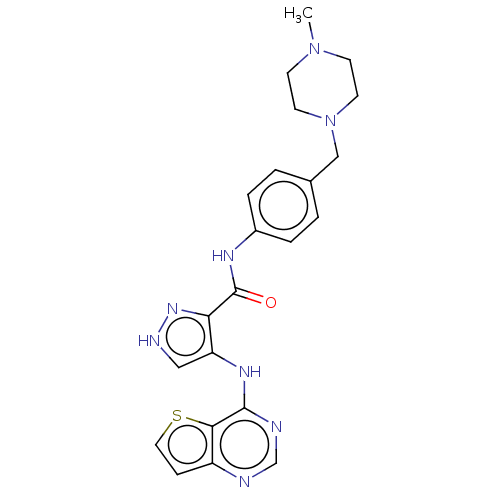
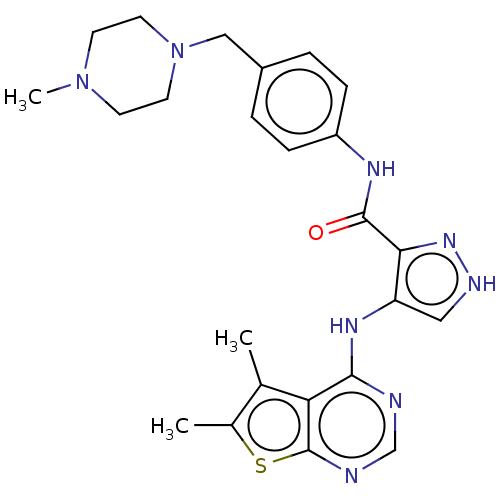
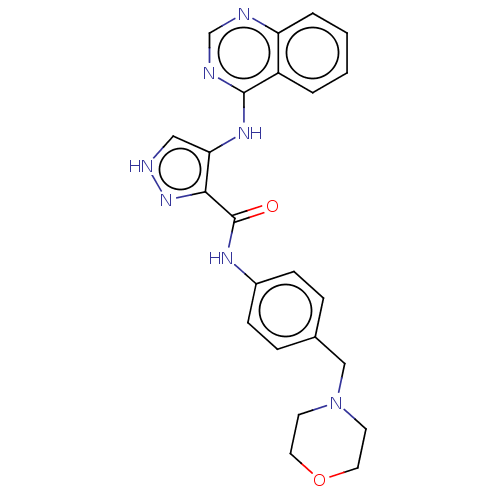
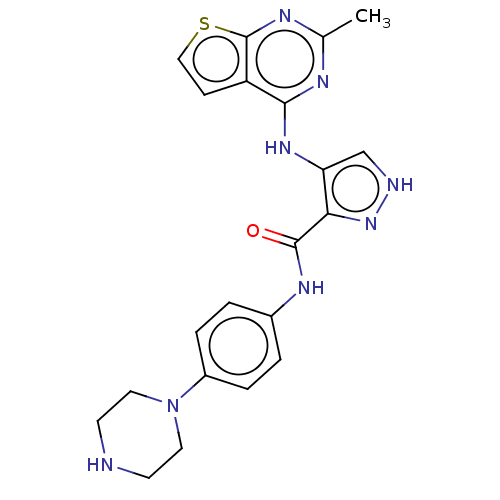
Affinity DataKi: 4.90nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 5.60nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Antagonistic activity against (-)-noradrenaline-induced contraction of rat prostatic vas deferens (Alpha-1A adrenergic receptor )More data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 42nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 65nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Inhibition of the cAMP-stimulated beta-galactosidase transcription in SK-N-MC cells expressing the human Histamine H3 receptorMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 300nMAssay Description:Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor)More data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 400nMAssay Description:Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 1.00E+3nMAssay Description:Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 1.30E+3nMAssay Description:Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atriumMore data for this Ligand-Target Pair
In DepthDetails
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.33E+3nMAssay Description:Competitive inhibition of PTP1B (unknown origin) using pNPP as substrate by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Antagonistic activity against (-)-noradrenaline-induced contraction of rat prostatic vas deferens (alpha1A receptor)More data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 5.00E+3nMAssay Description:Antagonistic activity against (-)-phenylephrine-induced contraction of rat spleen (Alpha-1B adrenergic receptor )More data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 6.90E+3nMAssay Description:Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor)More data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 8.00E+3nMAssay Description:Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atriumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 9.80E+3nMAssay Description:Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 1.20E+4nMAssay Description:Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 1.20E+4nMAssay Description:Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atriumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 1.57E+4nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 1.60E+4nMAssay Description:Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor)More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nMAssay Description:Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atriumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 2.00E+4nMAssay Description:Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 2.12E+4nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 2.70E+4nMAssay Description:Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor)More data for this Ligand-Target Pair
In DepthDetails
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.0350nMAssay Description:Inhibition of recombinant human full length VEGFR2 using poly (Glu, Tyr) as substrate by alpha screen assayMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.101nMAssay Description:Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.167nMAssay Description:Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:Inhibition of human FLT3 by Hotspot assayMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.270nMAssay Description:Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.320nMAssay Description:Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.327nMAssay Description:Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assayMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.340nMAssay Description:Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.384nMAssay Description:Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.438nMAssay Description:Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.470nMAssay Description:Inhibition of human FLT3 by Hotspot assayMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.470nMAssay Description:Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.540nMAssay Description:Inhibition of human FLT3 D835Y mutant by Hotspot assayMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.576nMAssay Description:Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.588nMAssay Description:Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human FLT3 by Hotspot assayMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.620nMAssay Description:Inhibition of human FLT3 by Hotspot assayMore data for this Ligand-Target Pair

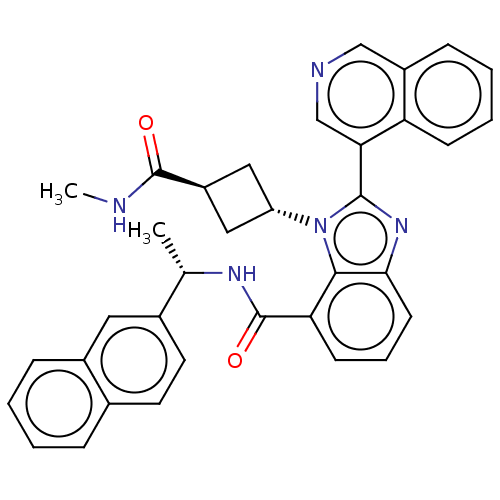

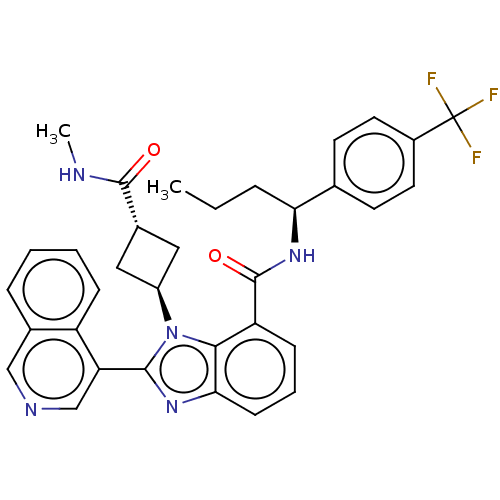
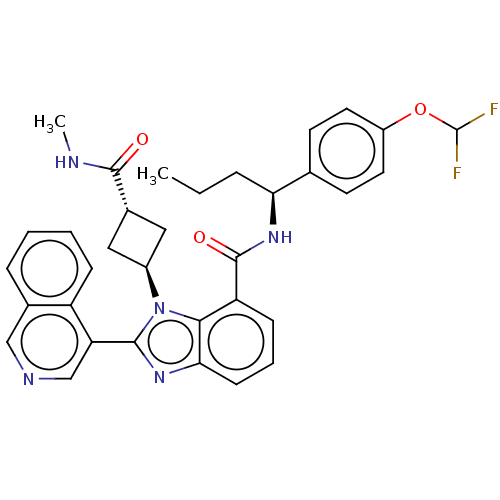
 3D Structure (crystal)
3D Structure (crystal)