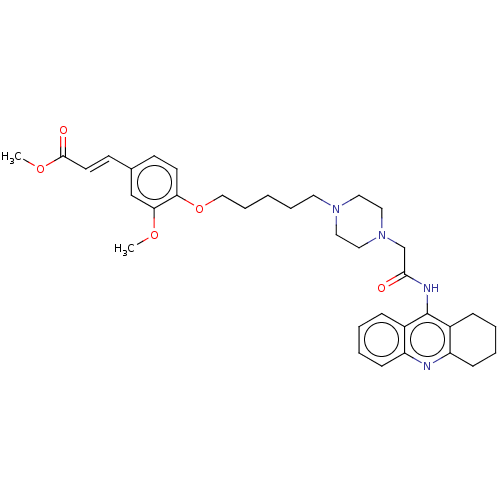
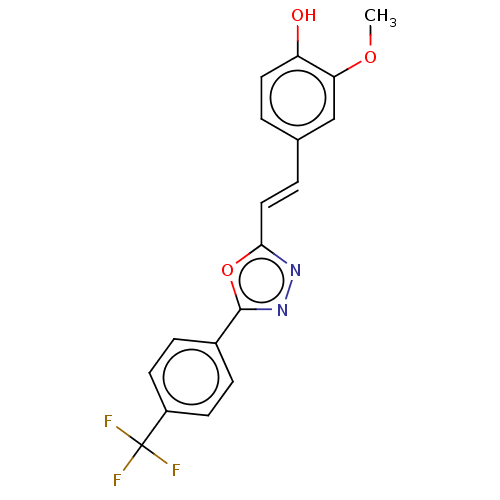
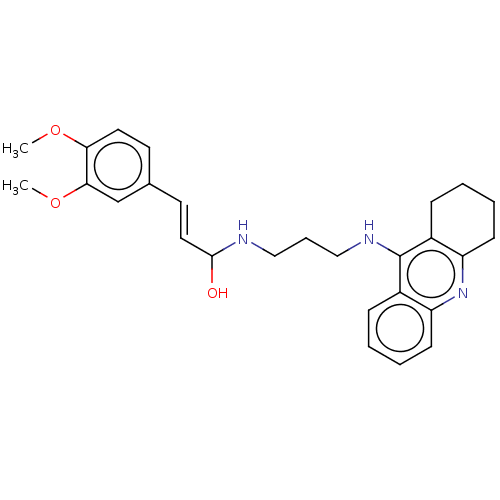
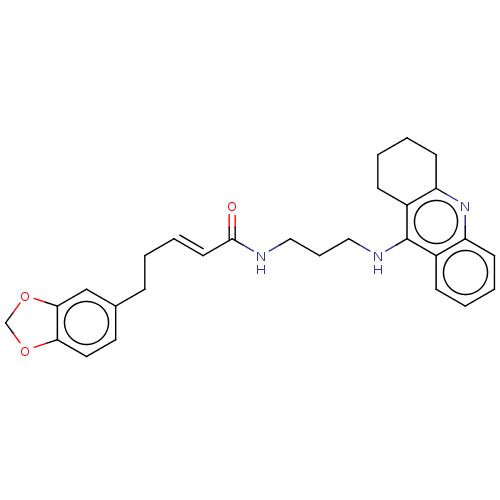
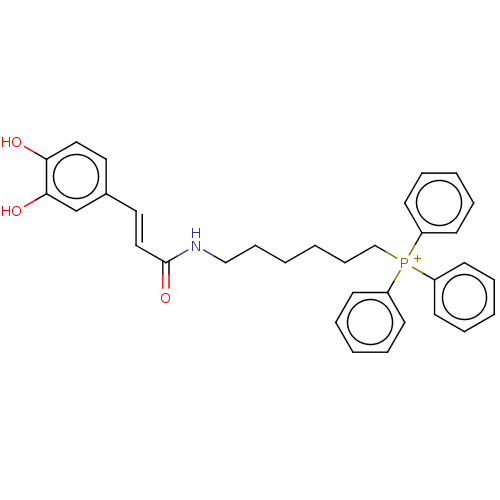
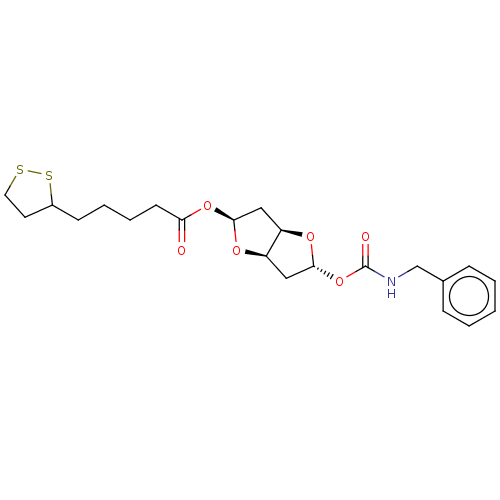
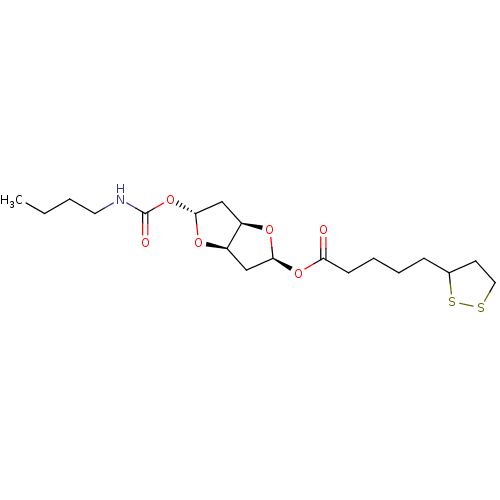
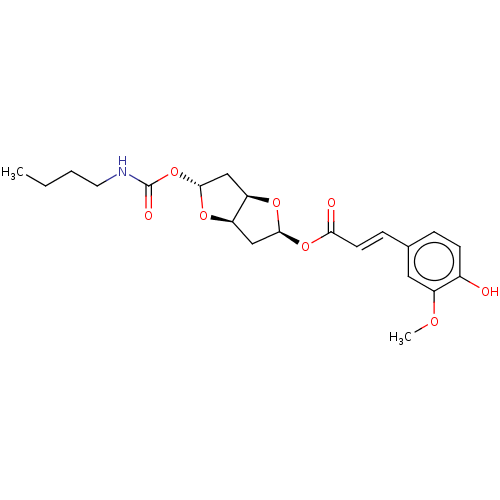
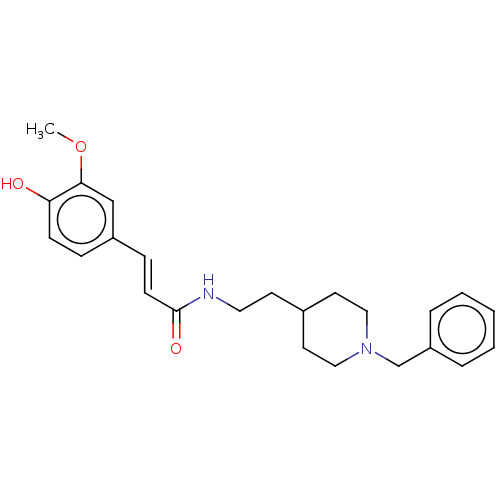
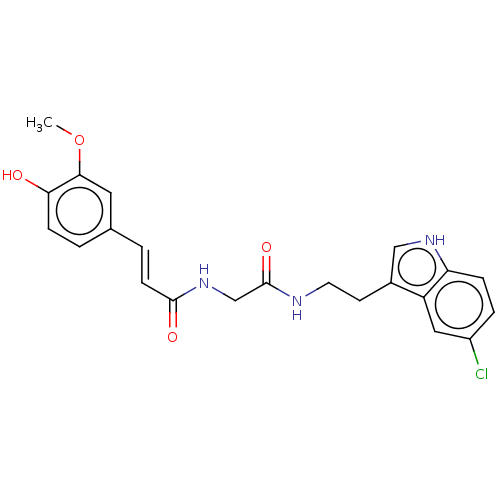
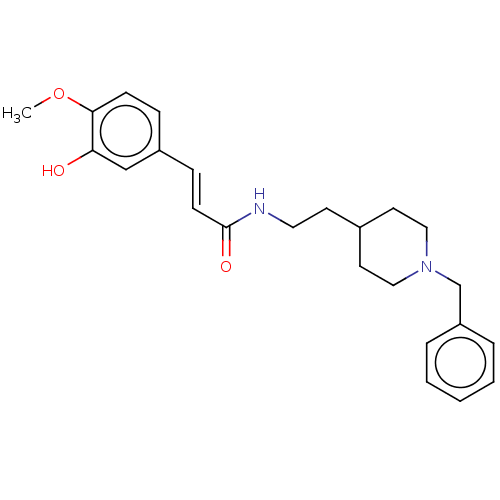
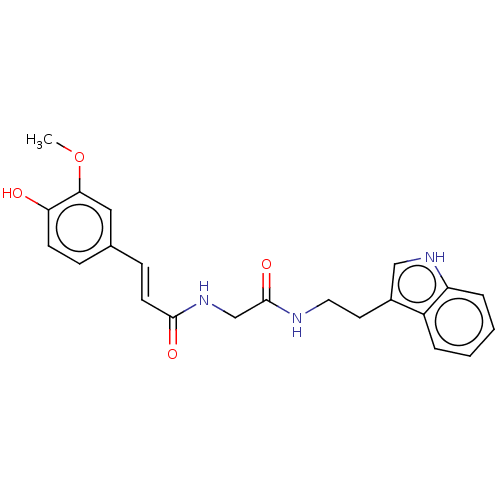
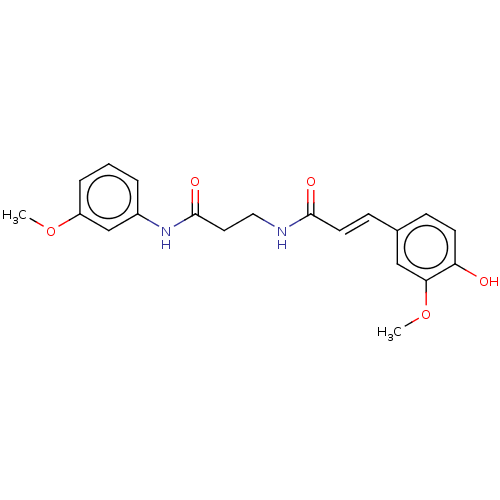
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition ...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 840nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 960nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 1.23E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.29E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.42E+3nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 2.16E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.41E+3nMAssay Description:Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 2.42E+3nMAssay Description:Inhibition of recombinant human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition ...More data for this Ligand-Target Pair

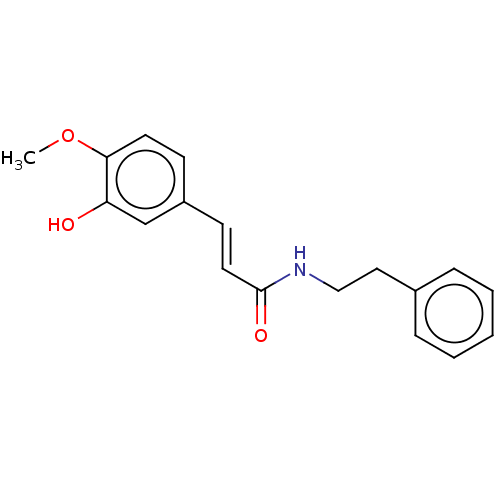


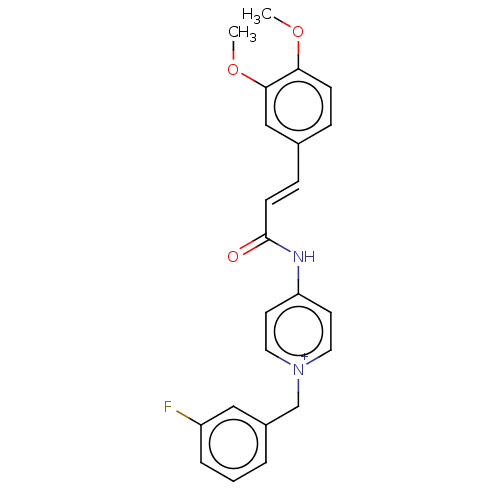
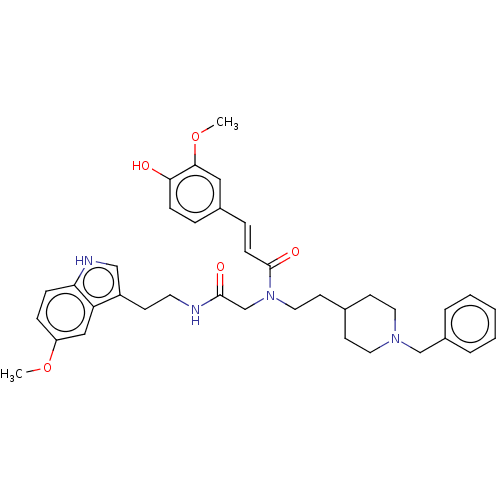
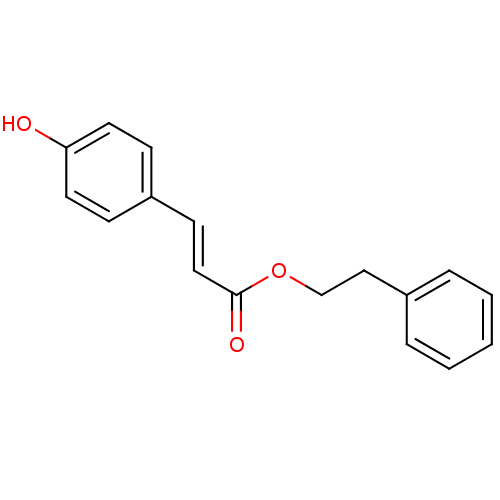
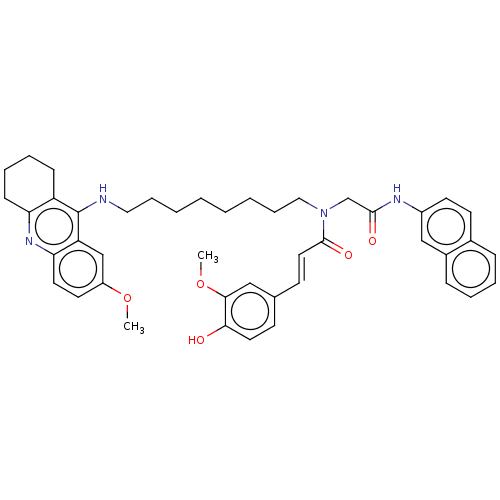

 3D Structure (crystal)
3D Structure (crystal)