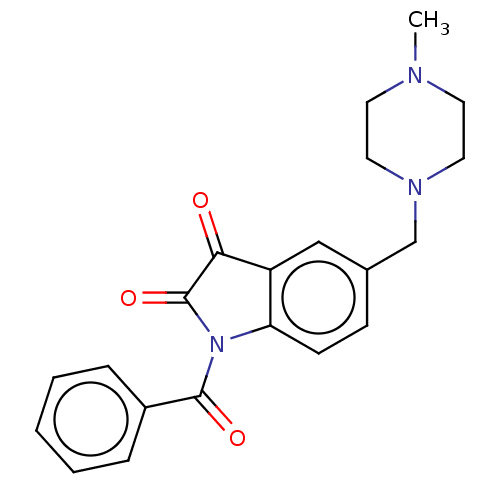
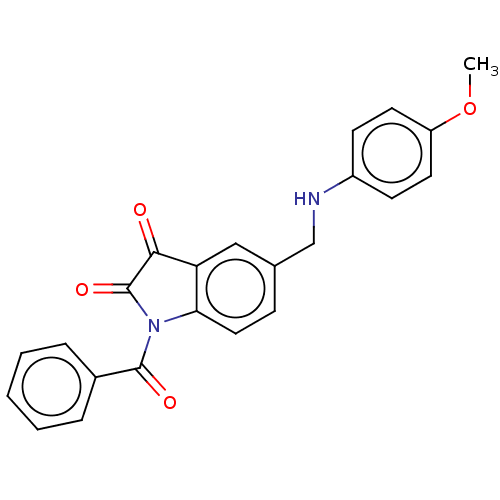
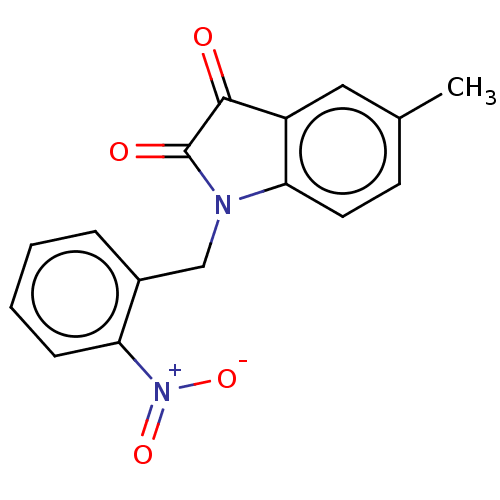
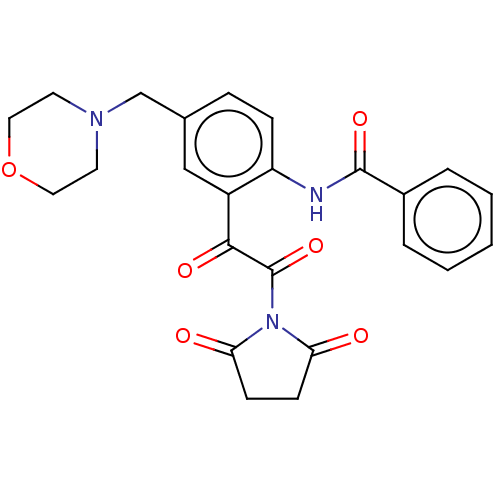
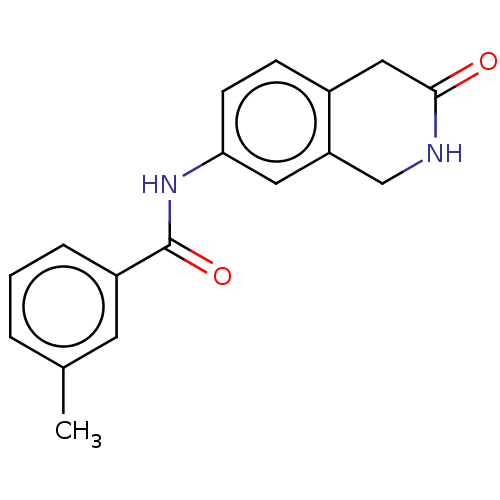
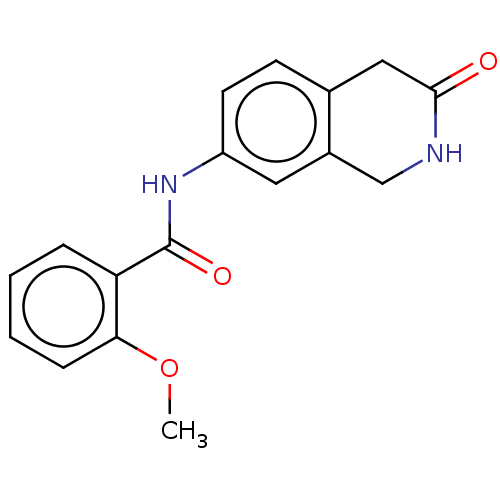
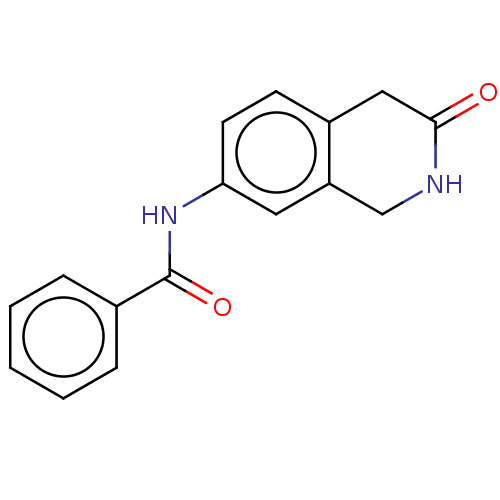
Affinity DataIC50: 0.0110nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0130nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0170nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0190nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0210nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0220nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0260nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0260nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0280nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0290nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.510nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 89nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 145nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 169nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 204nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 229nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 263nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 398nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 791nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 914nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 926nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.32E+3nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.36E+3nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of human PDE5A preincubated for 30 mins followed by TMB substrate addition by spectrophotometryMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Affinity DataIC50: 1.76E+4nMAssay Description:Inhibition of human PDE5A preincubated for 30 mins followed by TMB substrate addition by spectrophotometryMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human PDE5A preincubated for 30 mins followed by TMB substrate addition by spectrophotometryMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Affinity DataIC50: 9.12E+4nMAssay Description:Inhibition of human PDE5A preincubated for 30 mins followed by TMB substrate addition by spectrophotometryMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human PDE5A preincubated for 30 mins followed by TMB substrate addition by spectrophotometryMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human PDE5A preincubated for 30 mins followed by TMB substrate addition by spectrophotometryMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Affinity DataIC50: 1.31E+5nMAssay Description:Inhibition of human PDE5A preincubated for 30 mins followed by TMB substrate addition by spectrophotometryMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Affinity DataIC50: 1.58E+5nMAssay Description:Inhibition of human PDE5A preincubated for 30 mins followed by TMB substrate addition by spectrophotometryMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of human PDE5A preincubated for 30 mins followed by TMB substrate addition by spectrophotometryMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Affinity DataIC50: 7.46E+5nMAssay Description:Inhibition of human PDE5A preincubated for 30 mins followed by TMB substrate addition by spectrophotometryMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Department of Pharmaceutical Engineering, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China.
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of human PDE5A preincubated for 30 mins followed by TMB substrate addition by spectrophotometryMore data for this Ligand-Target Pair



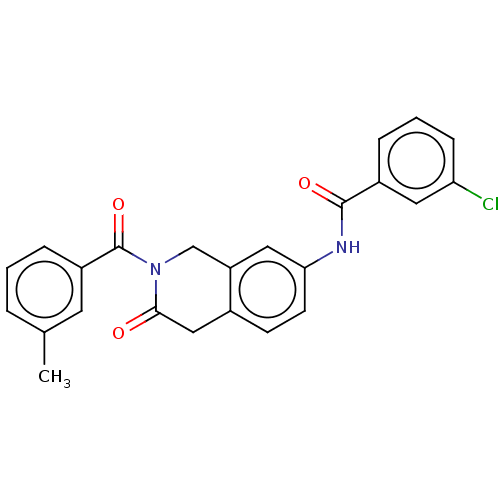

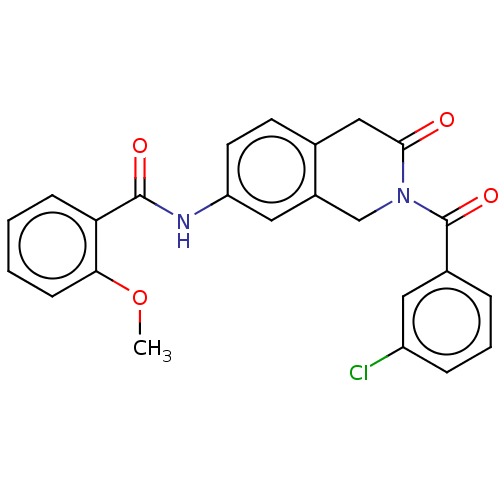
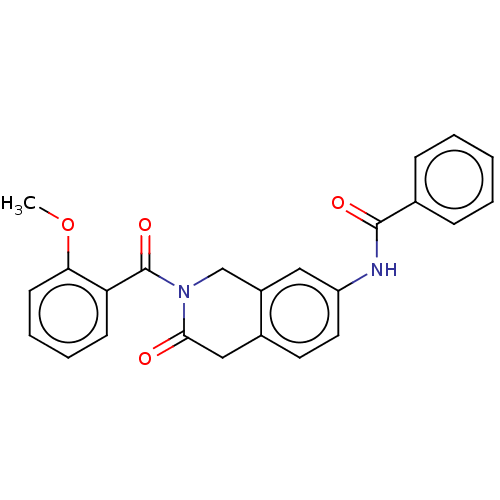
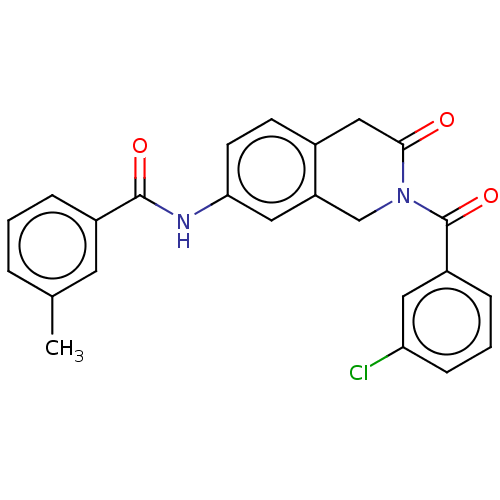
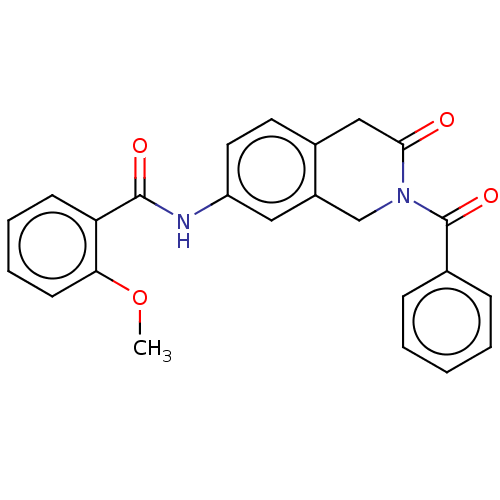
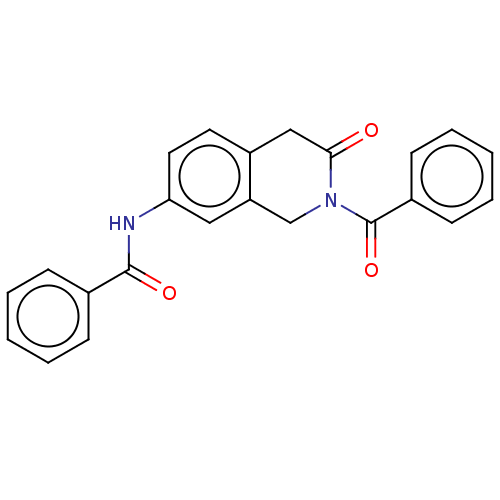

 3D Structure (crystal)
3D Structure (crystal)