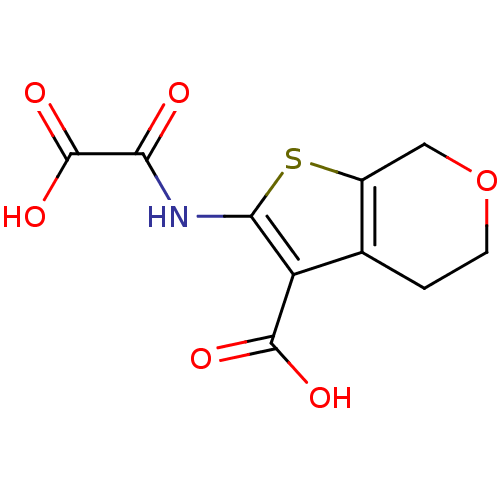
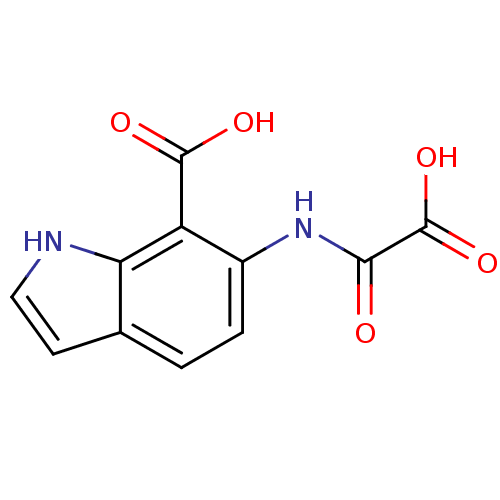
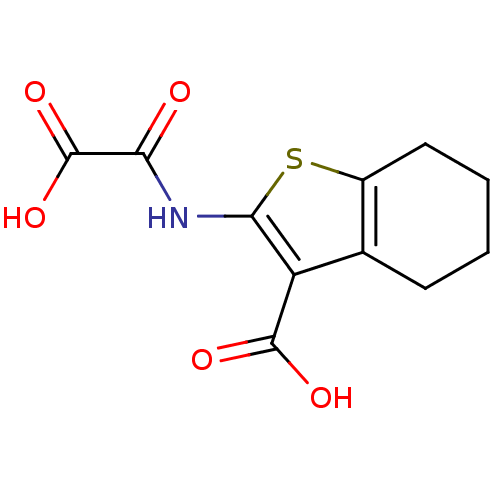
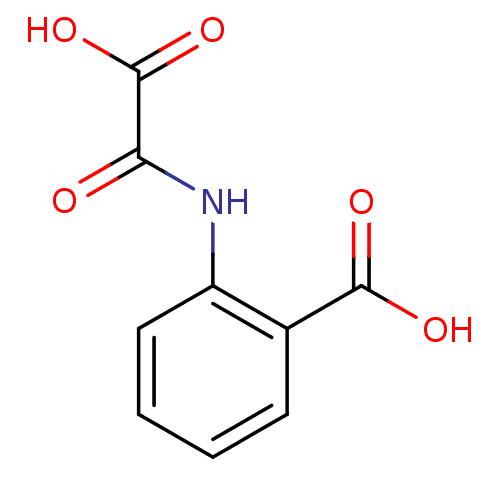
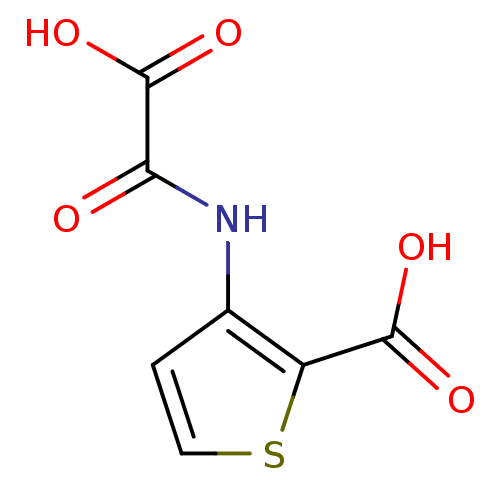
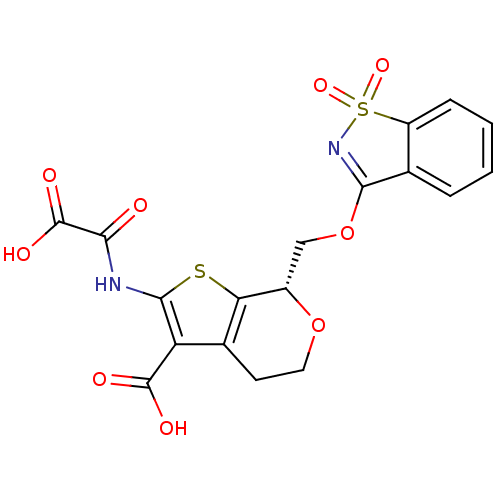
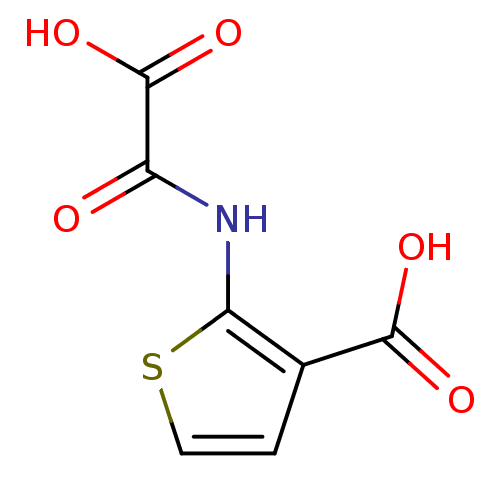
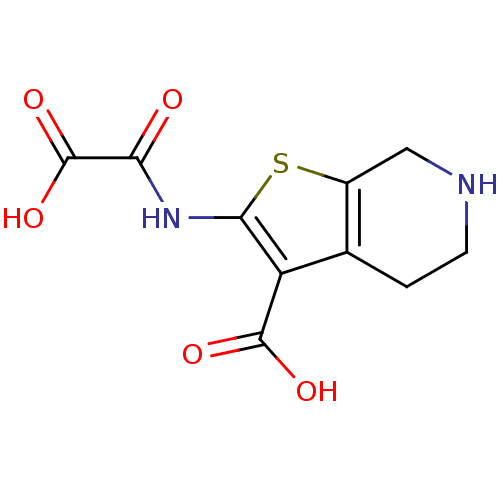
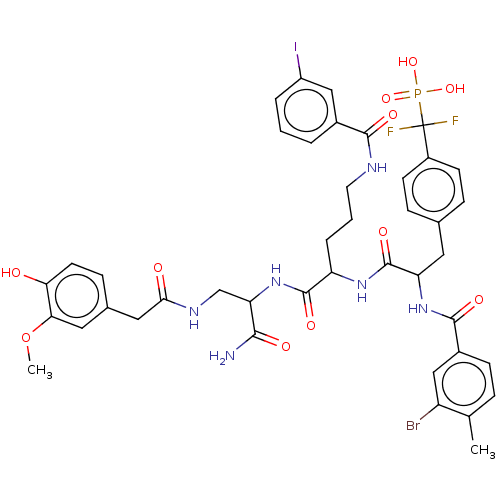
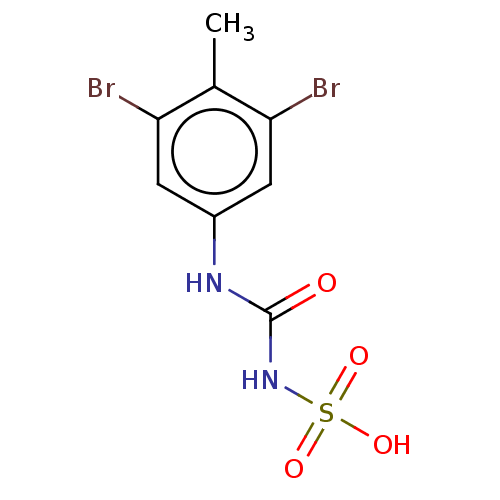
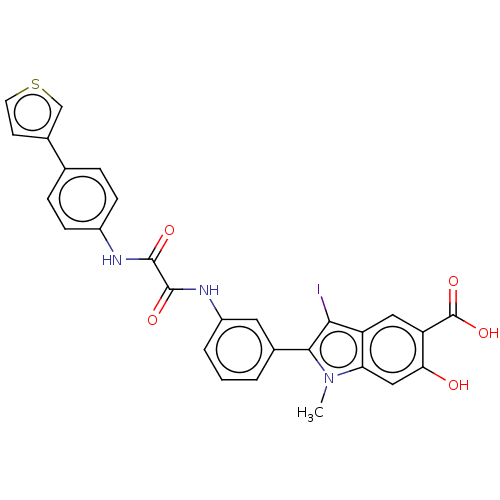
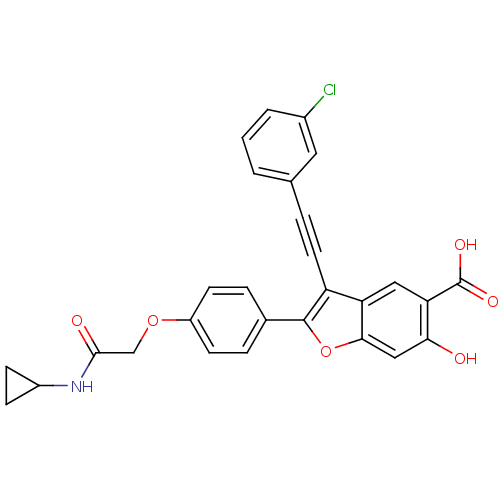
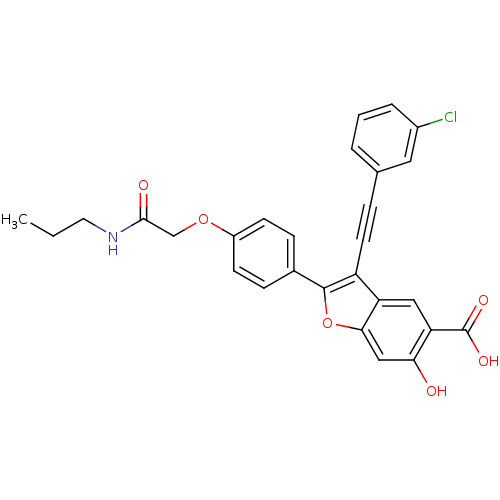
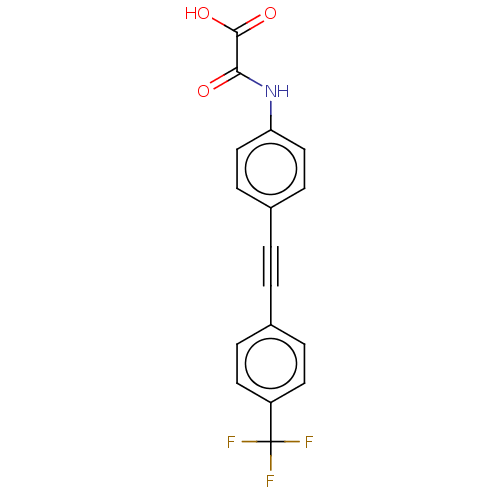
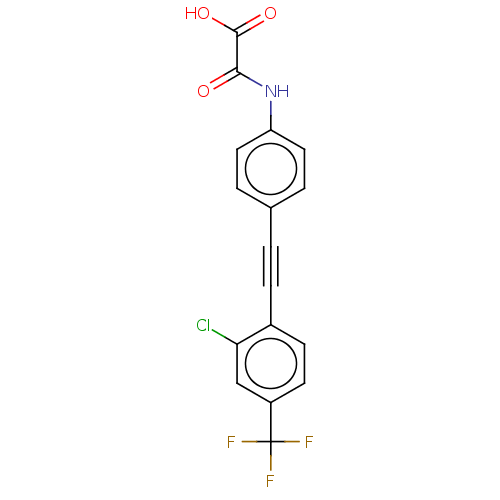
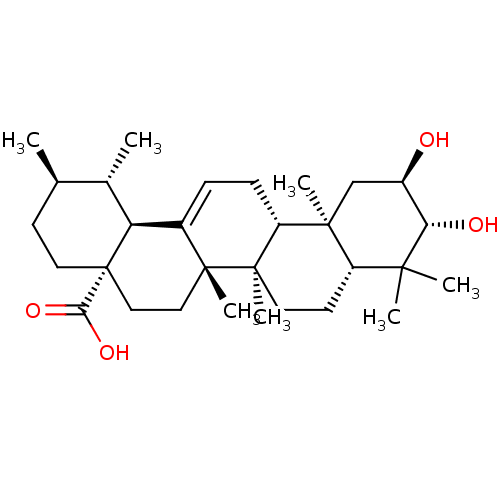
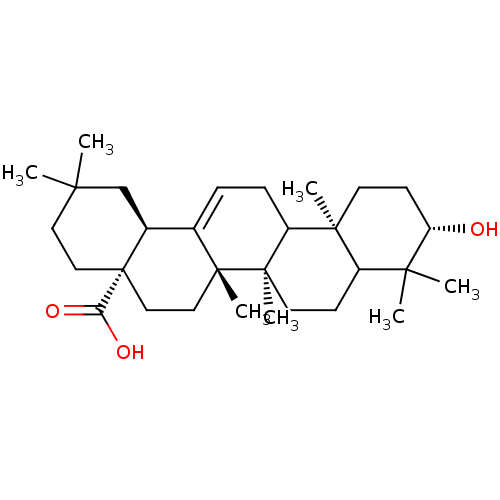
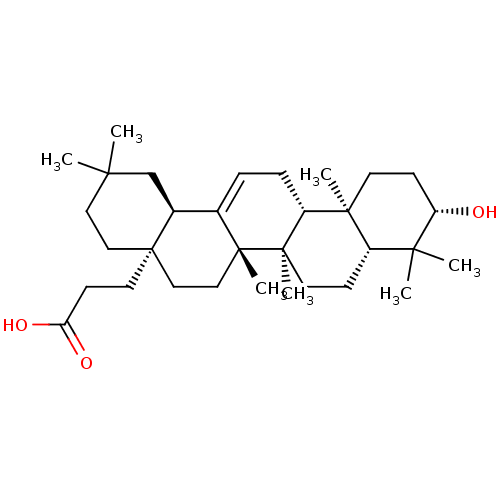
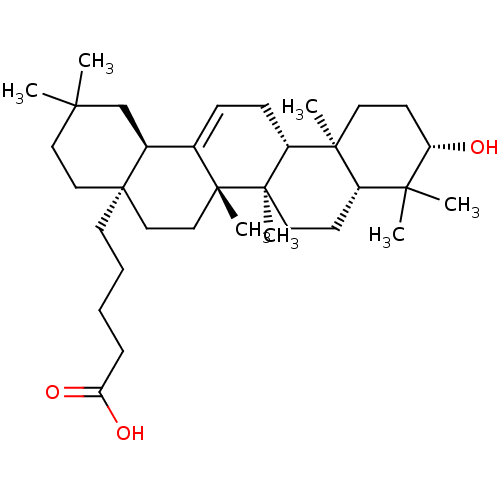
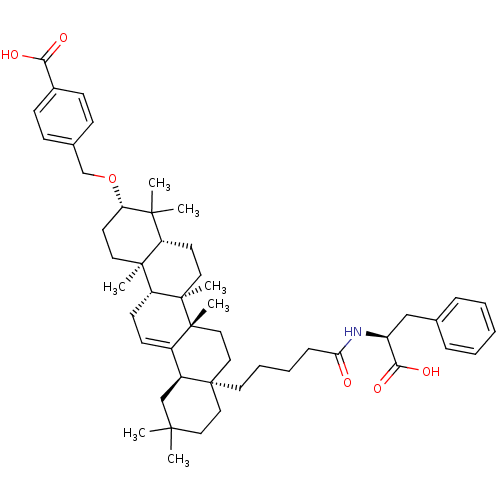
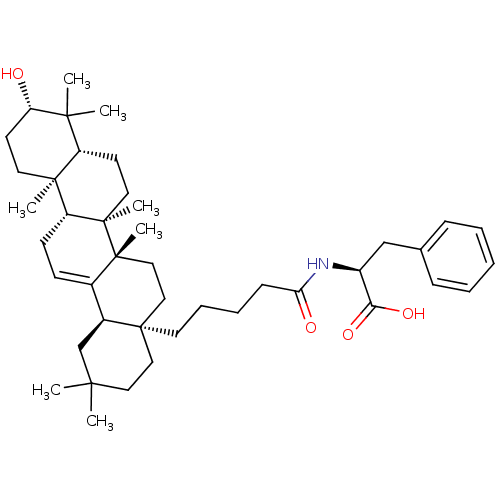
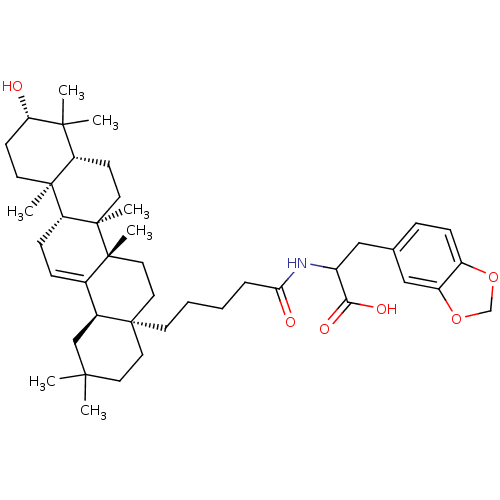
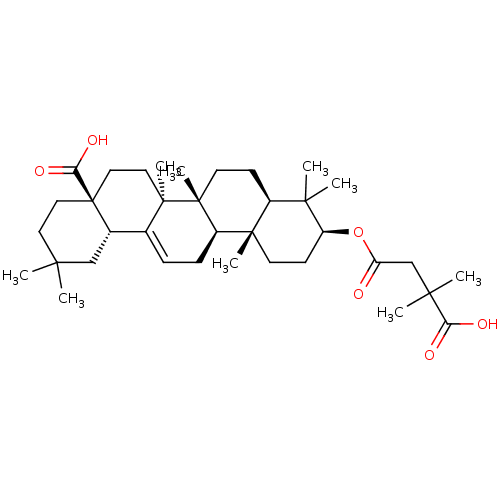
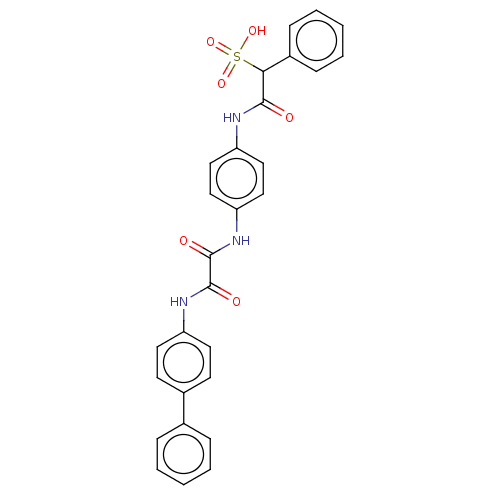
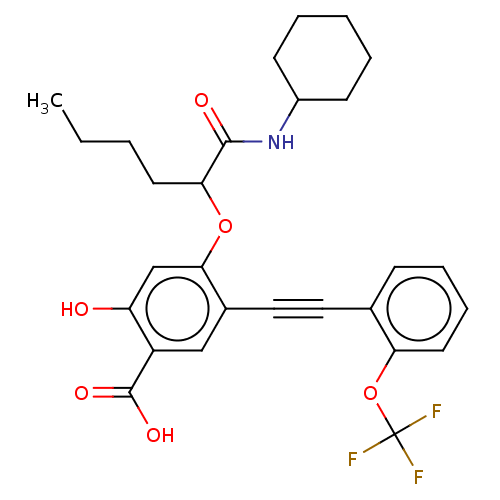
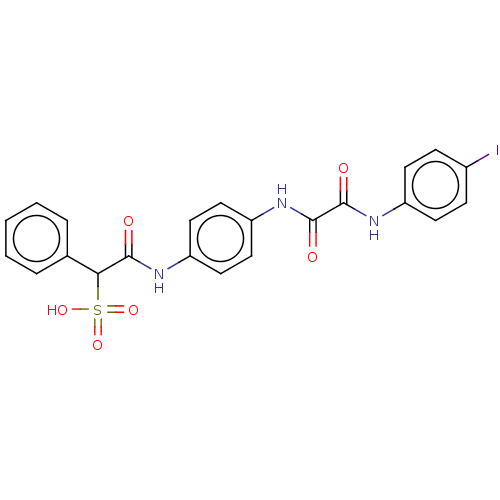
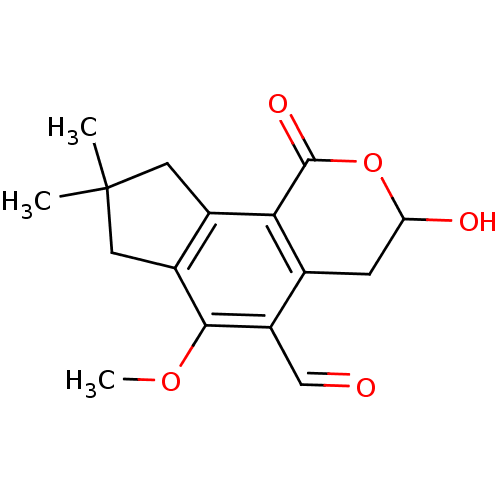
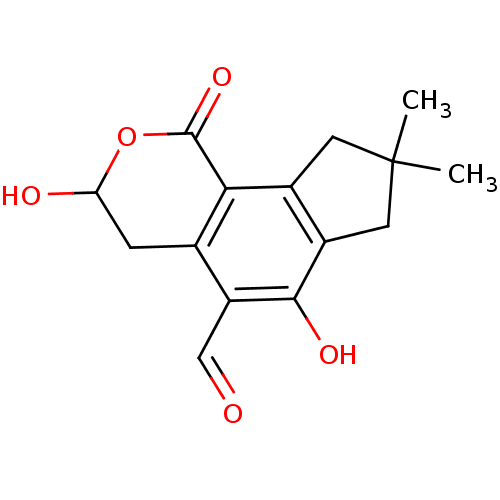
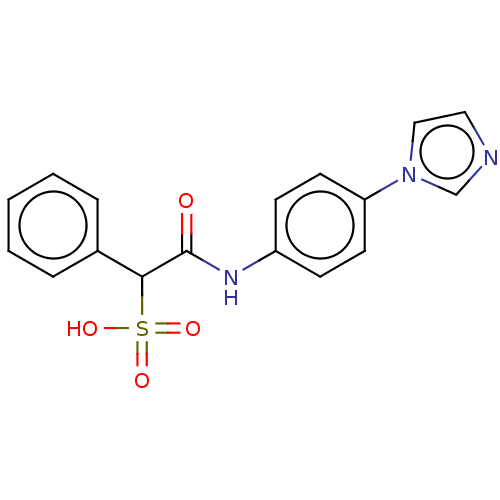
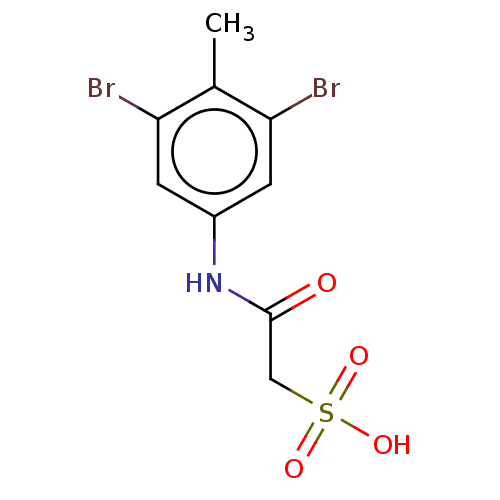
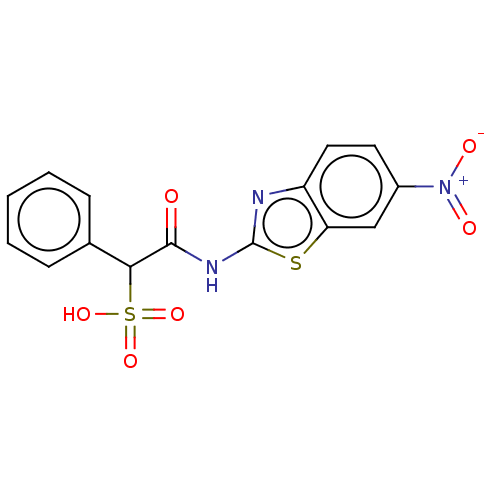
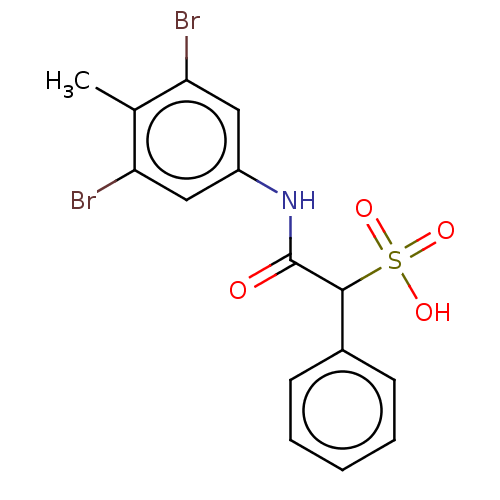
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 2.00E+4nMAssay Description:Inhibition of PTPREMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 3.30E+4nMAssay Description:Inhibition of PTPREMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 3.30E+4nMAssay Description:Inhibition of PTPREMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 4.50E+4nMAssay Description:Inhibition of PTPREMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 4.50E+4nMAssay Description:Inhibition of PTPREMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 6.60E+4nMAssay Description:Inhibition of PTPREMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 1.30E+5nMAssay Description:Inhibition of PTPREMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 3.60E+5nMAssay Description:Inhibition of PTPREMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: >5.00E+5nMAssay Description:Inhibition of PTPREMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 7.40E+5nMAssay Description:Inhibition of PTPREMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 1.10E+6nMAssay Description:Inhibition of PTPREMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 1.86E+3nMAssay Description:Inhibition of recombinant human cytosolic PTPepsilon expressed in Escherichia coli BL21 (DE3) using E527-P-Q-pY530-Q-P-G-E-N-L536 as substrate after ...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 2.01E+3nMAssay Description:Inhibition of recombinant human cytosolic PTPepsilon expressed in Escherichia coli BL21 (DE3) using E527-P-Q-pY530-Q-P-G-E-N-L536 as substrate after ...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 2.47E+3nMAssay Description:Inhibition of recombinant human cytosolic PTPepsilon expressed in Escherichia coli BL21 (DE3) using E527-P-Q-pY530-Q-P-G-E-N-L536 as substrate after ...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of recombinant human cytosolic PTPepsilon expressed in Escherichia coli BL21 (DE3) using E527-P-Q-pY530-Q-P-G-E-N-L536 as substrate after ...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.17E+3nMAssay Description:Inhibition of recombinant human cytosolic PTPepsilon expressed in Escherichia coli BL21 (DE3) using E527-P-Q-pY530-Q-P-G-E-N-L536 as substrate after ...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMpH: 7.0Assay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 ...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of PTPepsilon (unknown origin)More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:For selectivity studies, the PTPs, including LYP, mPTPA, SHP1-D1C, PTP1B, LMPTP, VHR, Laforin and PTPα-D1D2 were expressed and purified from E. ...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of recombinant PTPepsilon (unknown origin) using pNPP as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of recombinant PTPepsilon (unknown origin) using pNPP as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of PTPE (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of PTPE (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant PTPepsilonMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant PTPepsilonMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant PTPepsilonMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant PTPepsilonMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant PTPepsilonMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant PTPepsilonMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant PTPepsilonMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant PTPepsilonMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant PTPepsilonMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant PTPepsilonMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant PTPepsilonMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of PTPE (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of phosphatase activity of human PTPepsilon using pNPP as a substrate after 10 mins by spectrophotometer analysisMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of phosphatase activity of human PTPepsilon using pNPP as a substrate after 10 mins by spectrophotometer analysisMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMpH: 7.0Assay Description:Inhibition of PTPepsilon (unknown origin) using pNPP as substrate at pH 7 at 25 degC by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of phosphatase activity of human PTPepsilon using pNPP as a substrate after 10 mins by spectrophotometer analysisMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of PTPep (unknown origin) using DiFMUP as substrate incubated for 30 mins followed by substrate addition at pH 6.5 by standard phosphatase...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of PTPep (unknown origin) using DiFMUP as substrate incubated for 30 mins followed by substrate addition at pH 6.5 by standard phosphatase...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of PTPep (unknown origin) using DiFMUP as substrate incubated for 30 mins followed by substrate addition at pH 6.5 by standard phosphatase...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of PTPep (unknown origin) using DiFMUP as substrate incubated for 30 mins followed by substrate addition at pH 6.5 by standard phosphatase...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase epsilon(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated...More data for this Ligand-Target Pair