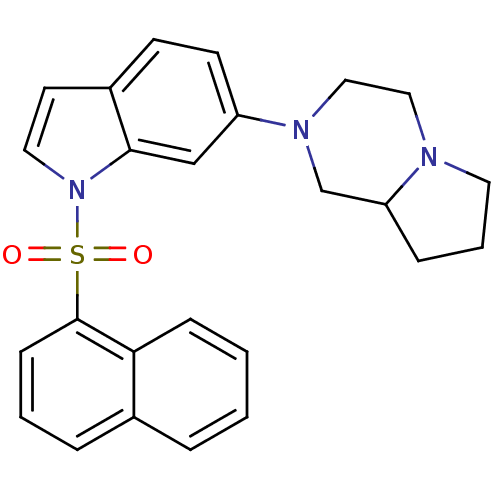
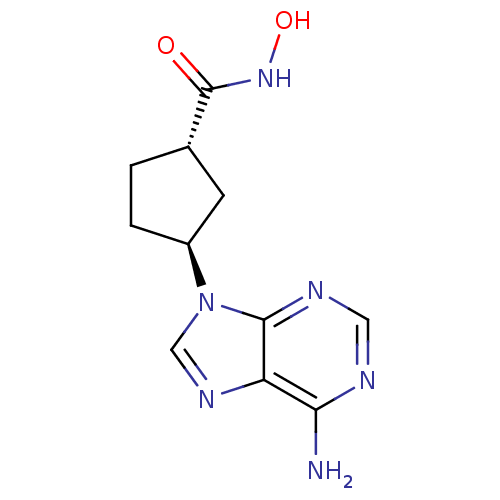
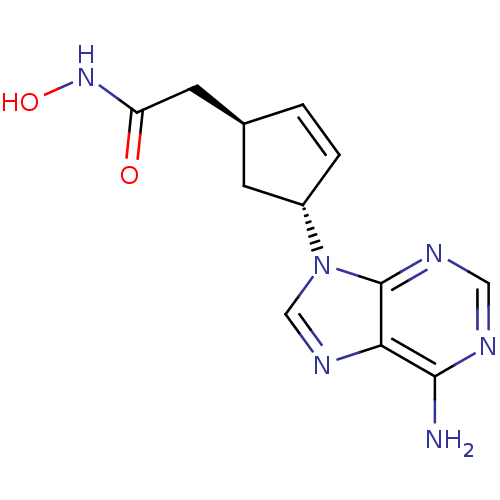
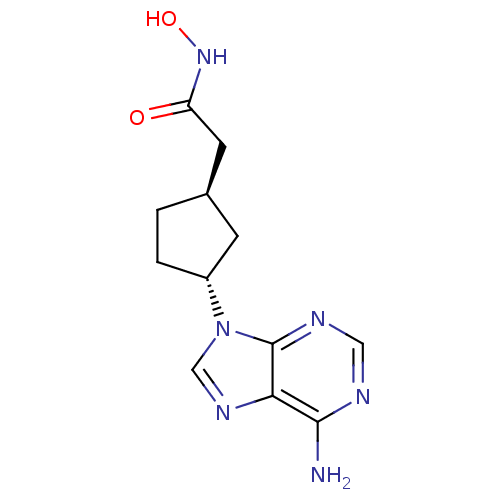
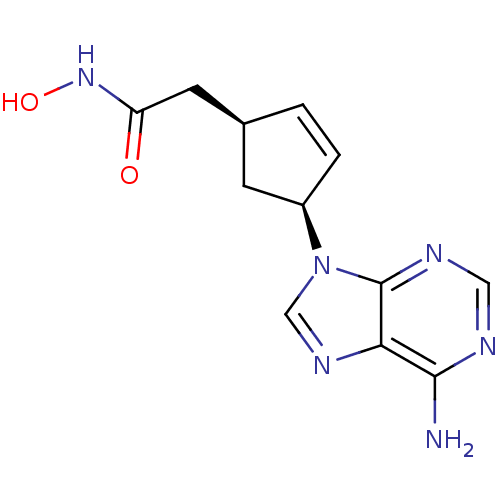
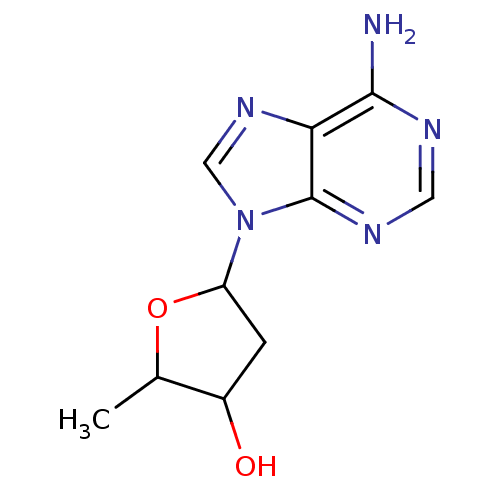
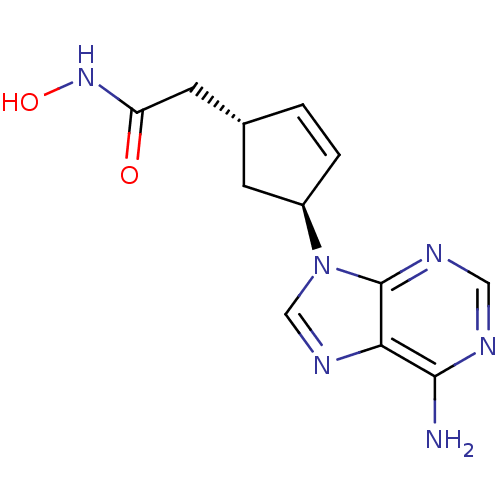
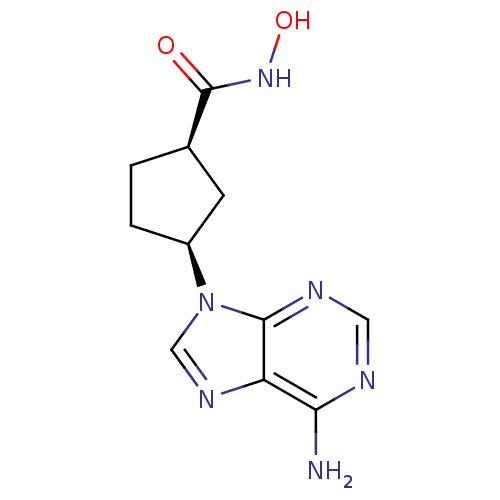
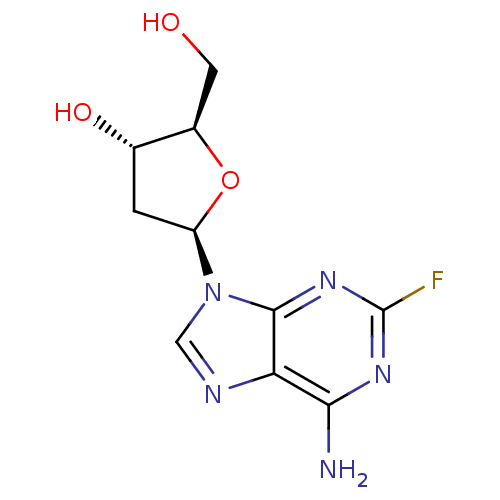
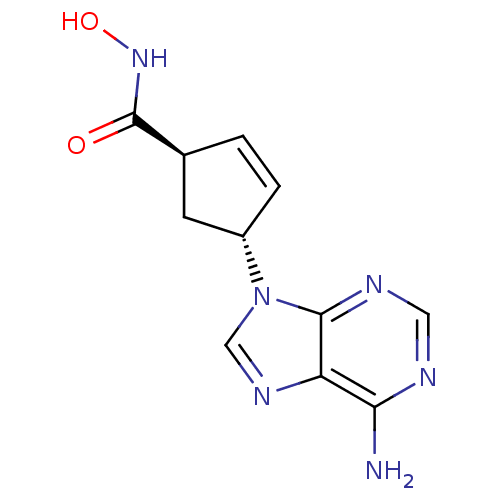
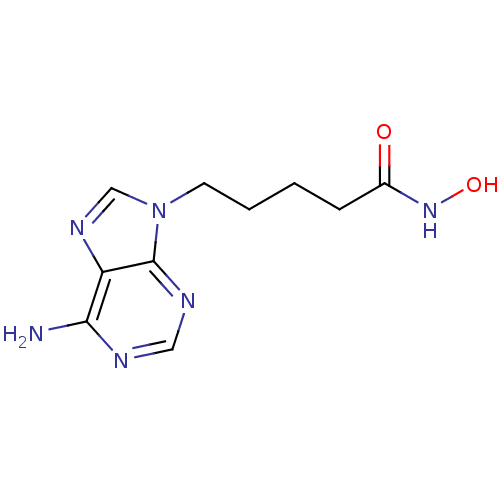
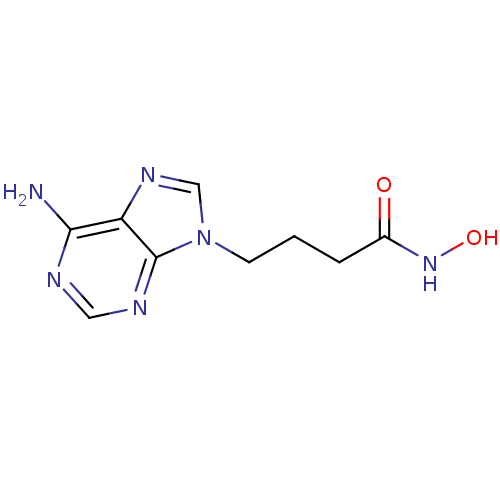
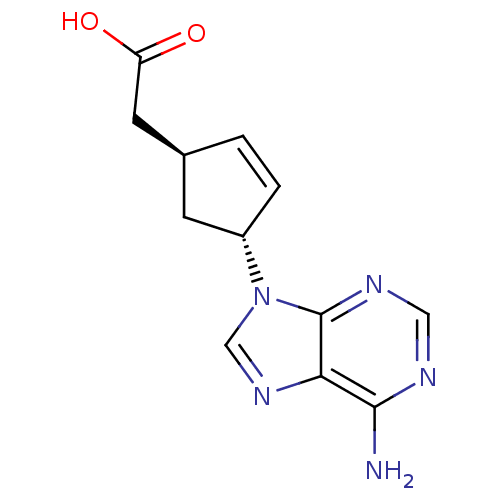
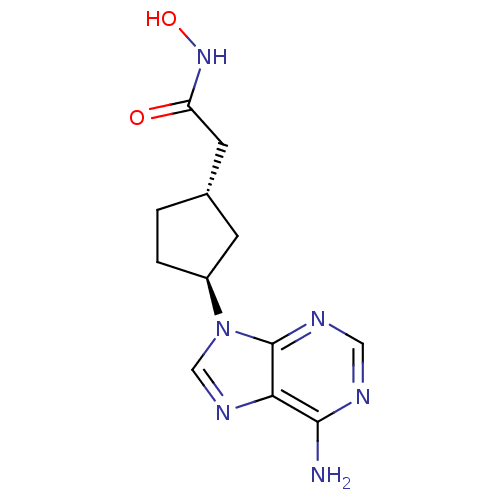
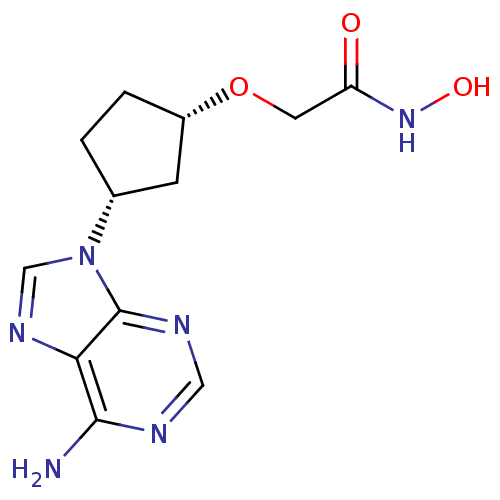
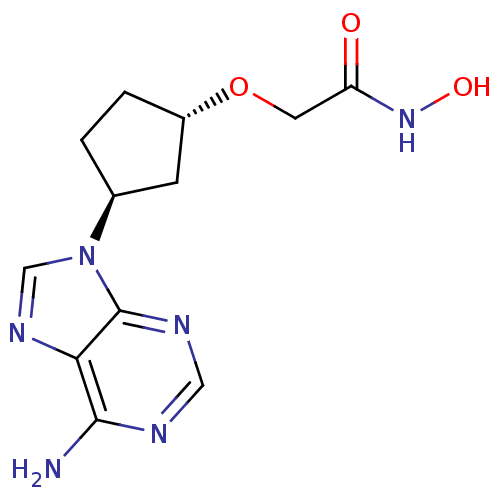
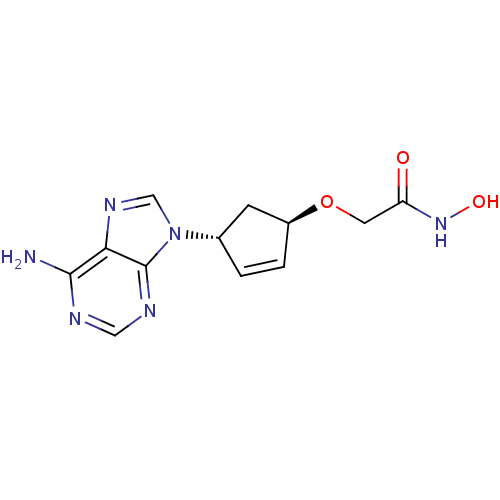
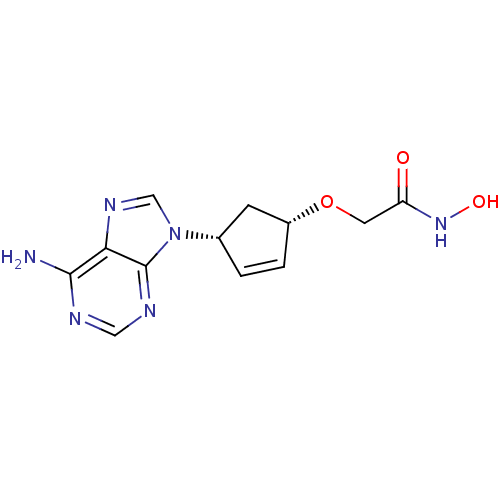
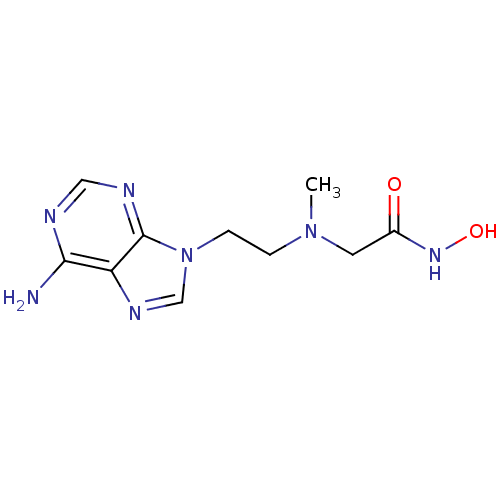
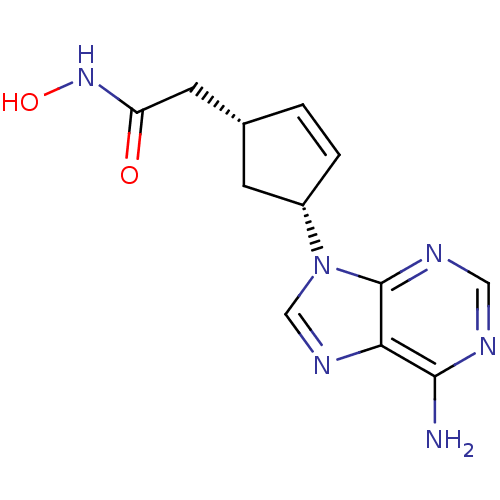
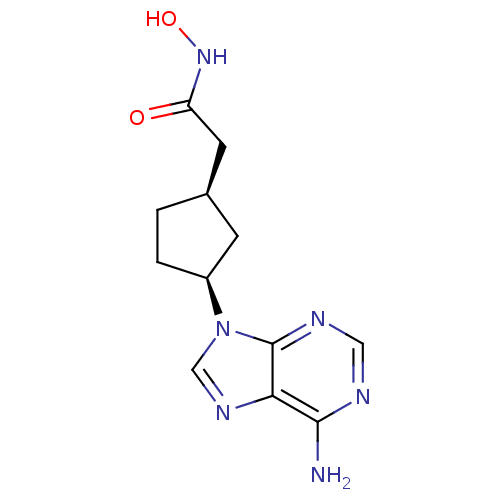
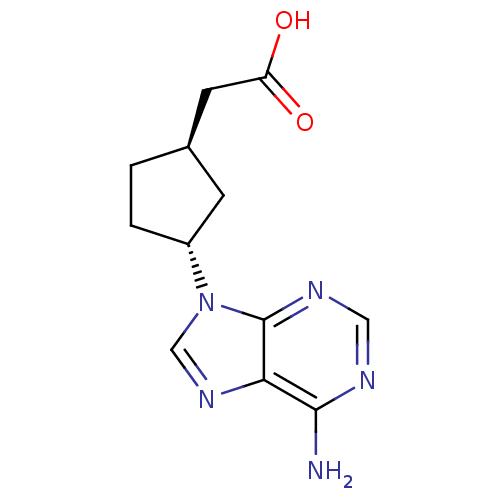
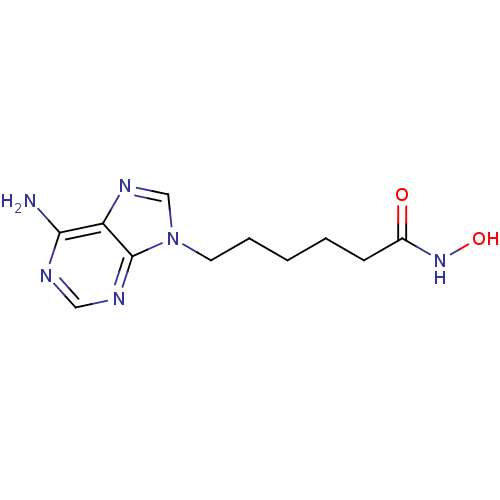
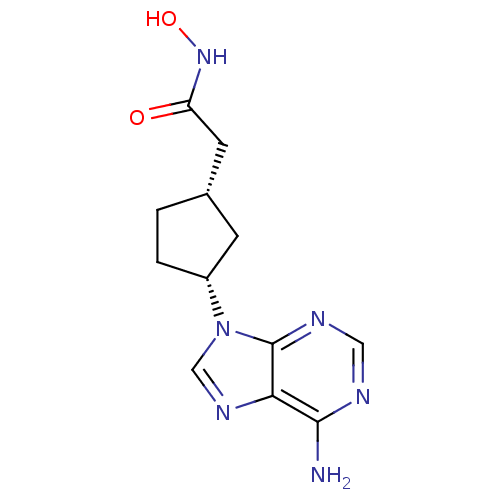
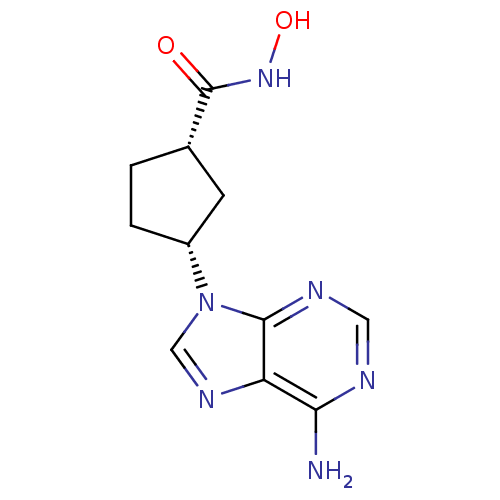
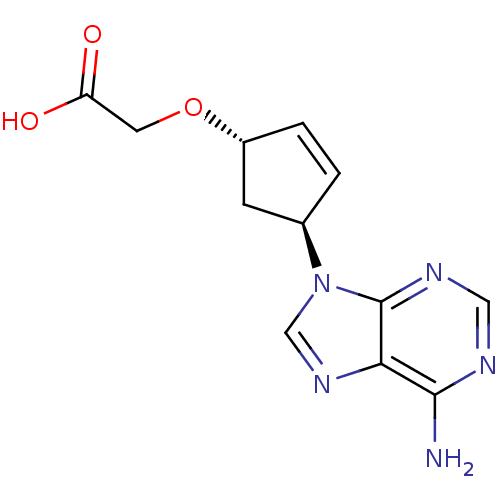
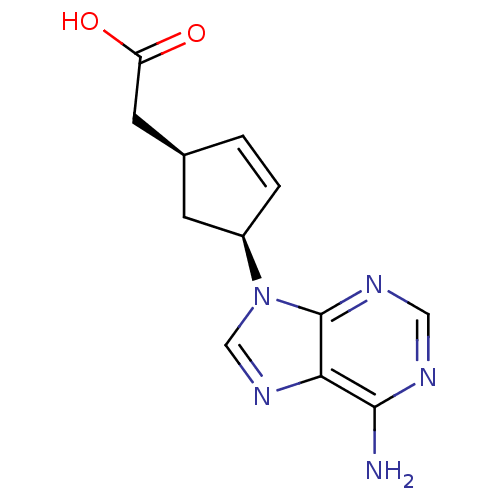
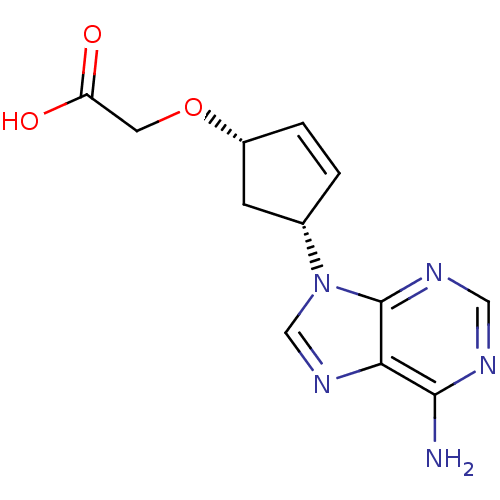
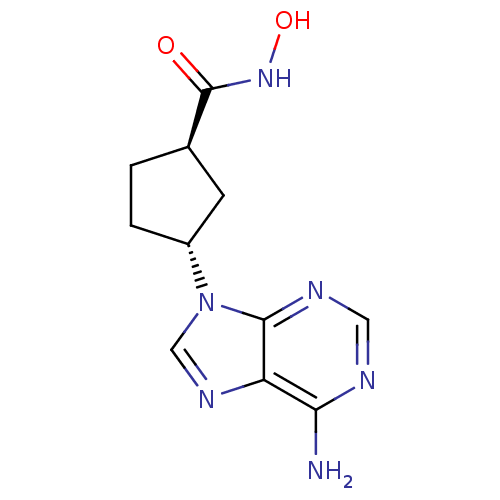
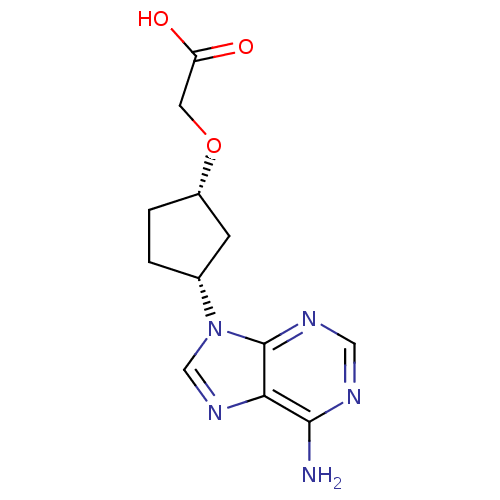
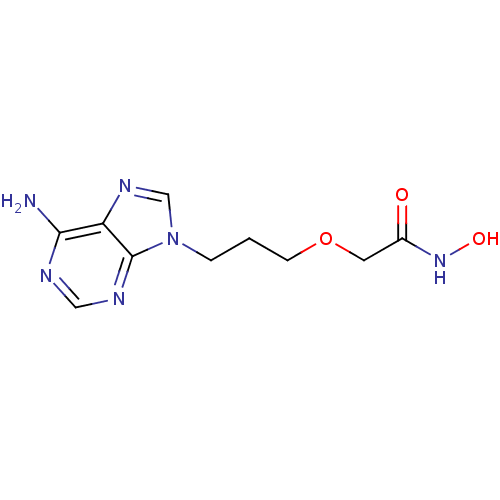
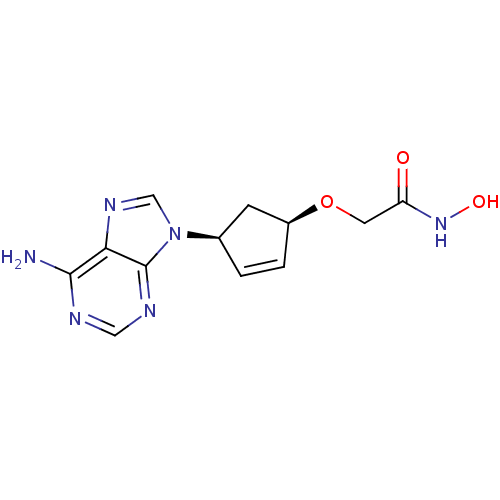
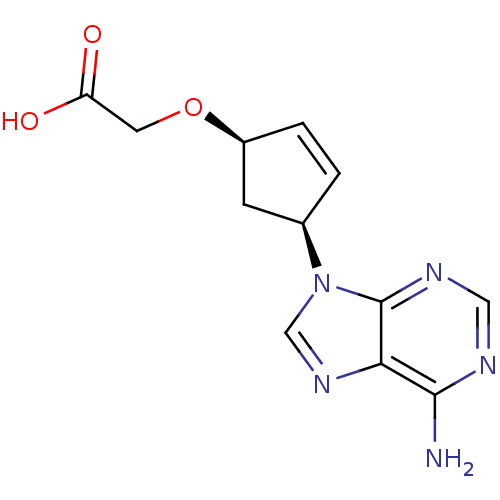
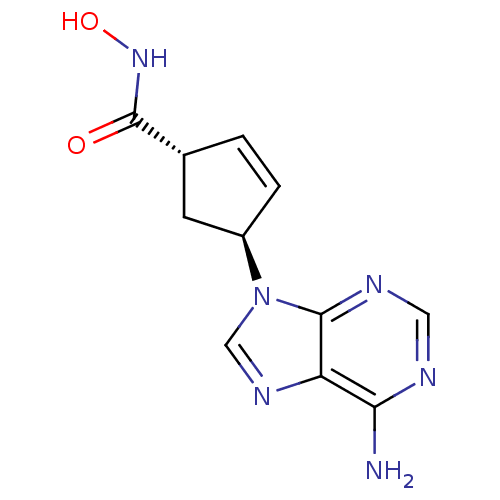
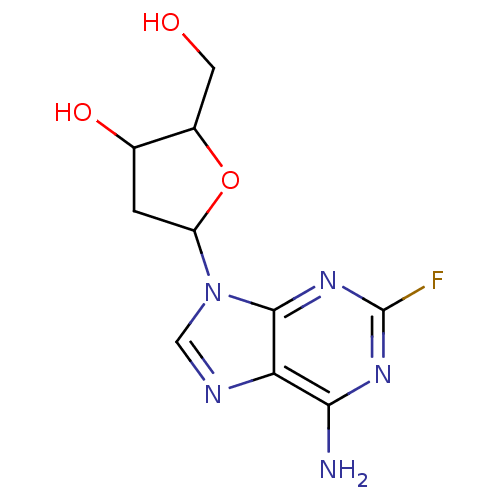
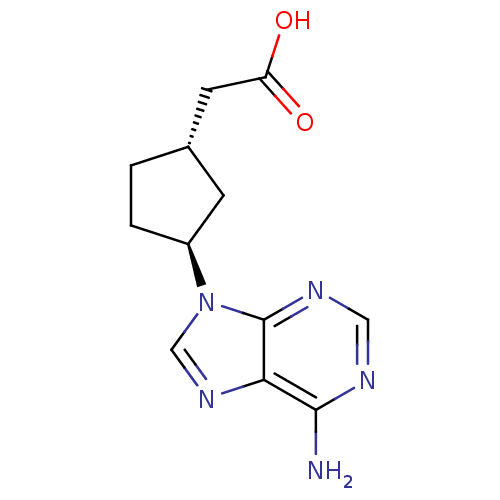
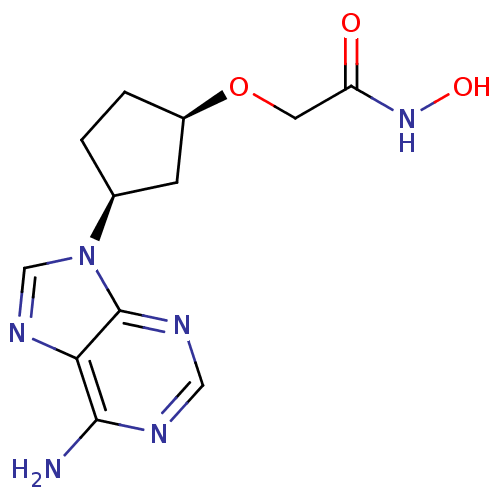
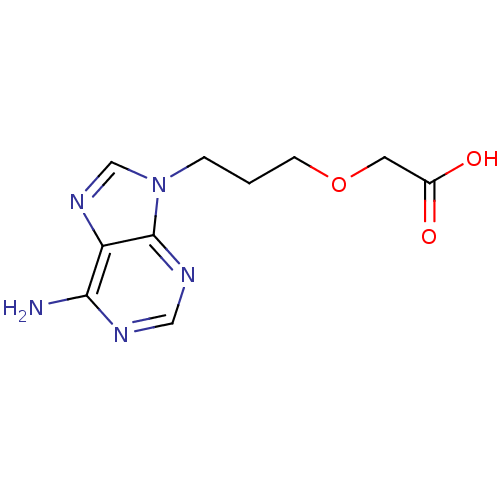
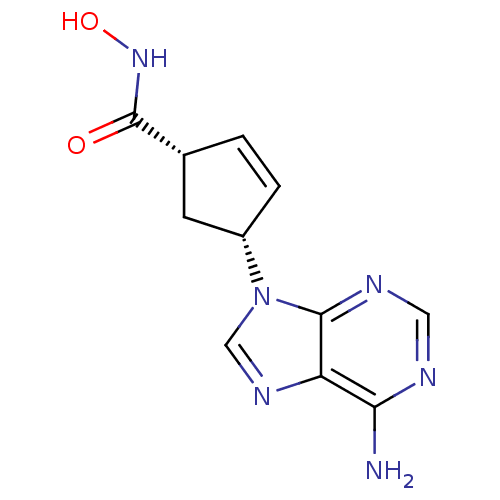
Affinity DataIC50: 7.20nMAssay Description:Antagonistic activity of the compound evaluated in adenylyl cyclase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
TargetAdenylate cyclase type 5(Rattus norvegicus)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Compound was evaluated for inhibition of adenylate cyclase from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 4.60E+3nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
TargetAdenylate cyclase type 5(Rattus norvegicus)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 4.60E+3nMAssay Description:Compound was evaluated for inhibition of adenylate cyclase from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibitory concentration of the compound against type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90E+3nMAssay Description:Inhibitory concentration of the compound against type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90E+3nMAssay Description:Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90E+3nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 8.20E+3nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 9.40E+3nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+4nMAssay Description:Inhibitory concentration of the compound against Type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+4nMAssay Description:Inhibitory concentration of the compound against Type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+4nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 1.36E+4nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 1.36E+4nMAssay Description:Inhibitory concentration of the compound against Type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.36E+4nMAssay Description:Inhibitory concentration of the compound against Type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 5(Rattus norvegicus)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Compound was evaluated for inhibition of adenylate cyclase from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.53E+4nMAssay Description:Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.63E+4nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 2.51E+4nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 2.88E+4nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 3.54E+4nMAssay Description:Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.54E+4nMAssay Description:Inhibitory concentration of the compound against type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 3.54E+4nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+4nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+4nMAssay Description:Inhibitory concentration of the compound against Type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 7.32E+4nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+4nMAssay Description:Inhibitory concentration of the compound against Type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+4nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 7.90E+4nMAssay Description:Inhibitory concentration of the compound against Type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 9.40E+4nMAssay Description:Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 9.40E+4nMAssay Description:Inhibitory concentration of the compound against type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 9.60E+4nMAssay Description:Inhibitory concentration of the compound against Type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 9.80E+4nMAssay Description:Inhibitory concentration of the compound against Type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
TargetAdenylate cyclase type 5(Rattus norvegicus)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Compound was evaluated for inhibition of adenylate cyclase from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+5nMAssay Description:Inhibitory concentration of the compound against Type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+5nMAssay Description:Inhibitory concentration of the compound against Type V Adenyl Cyclase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.41E+5nMAssay Description:Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.88E+5nMAssay Description:Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells.More data for this Ligand-Target Pair