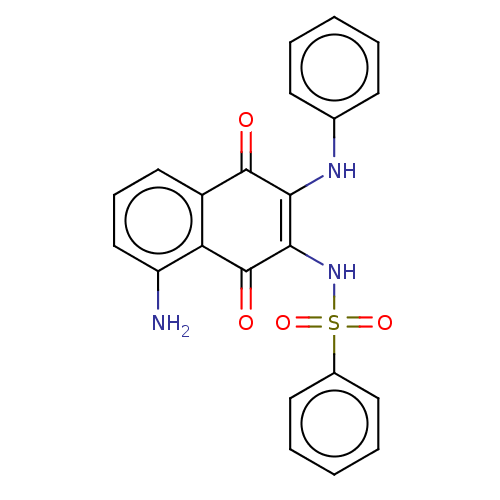
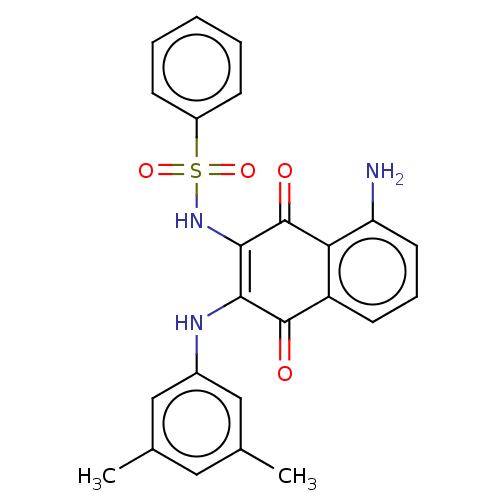
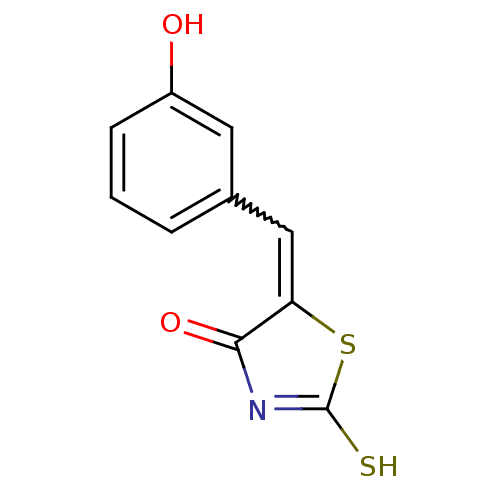
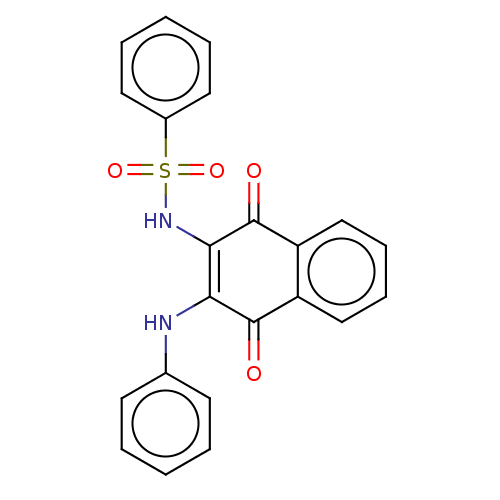
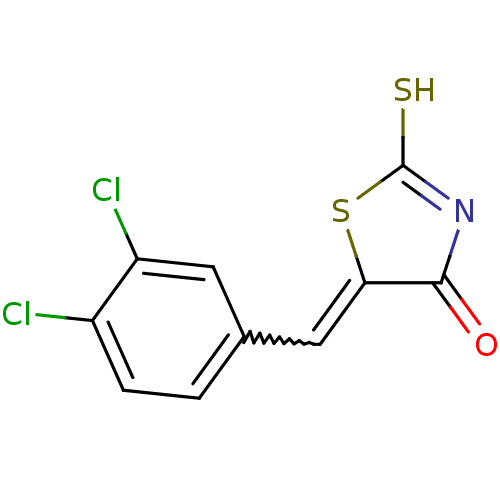
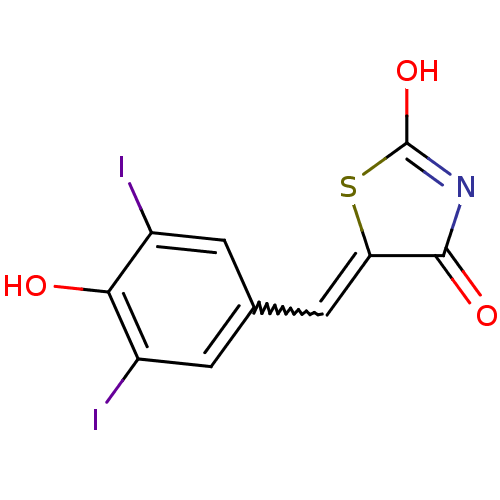
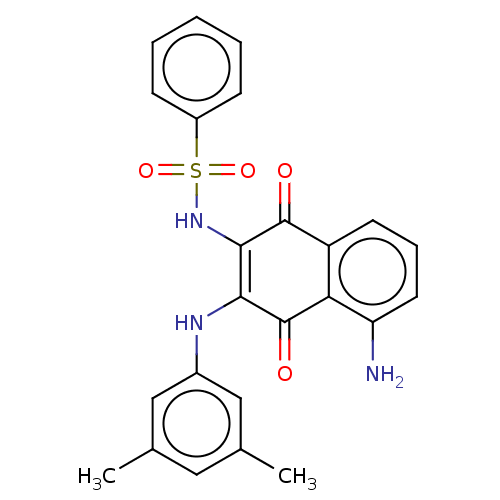
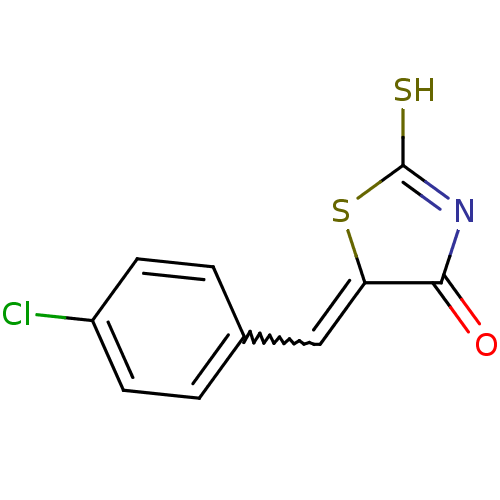
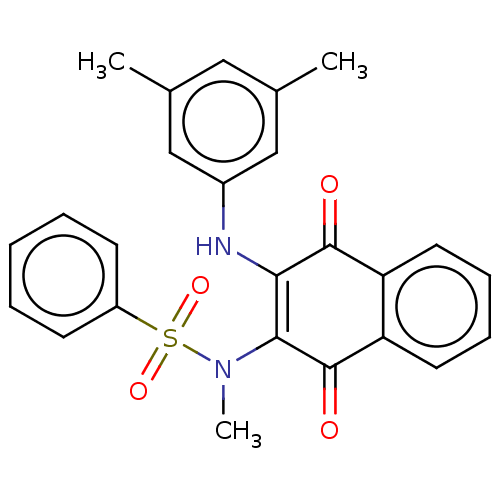
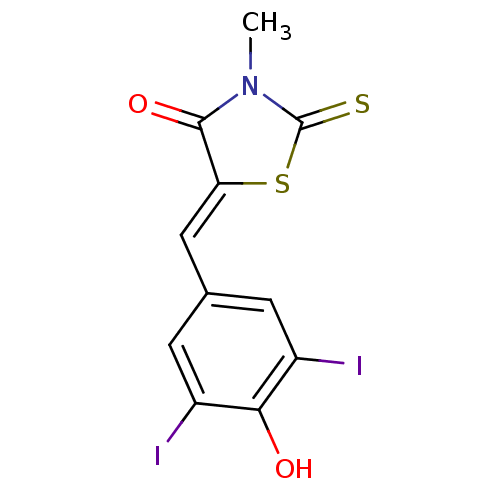
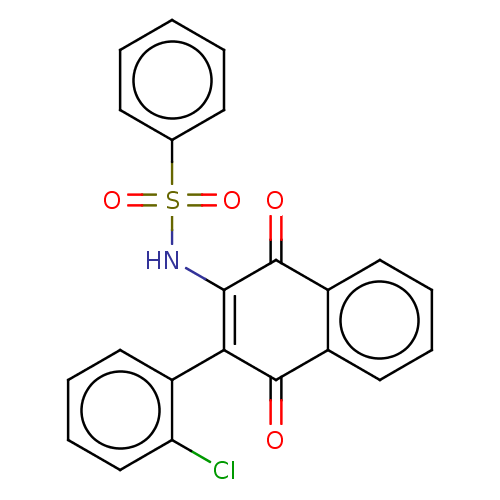
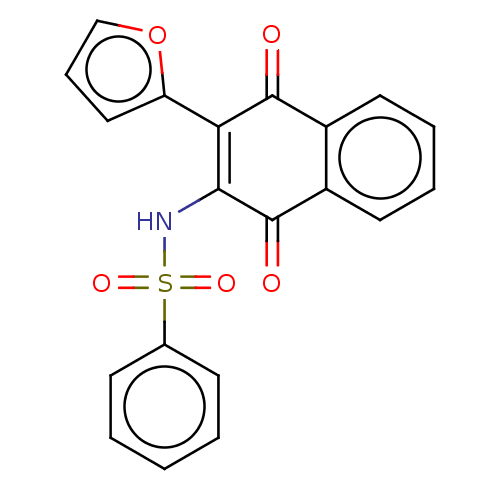
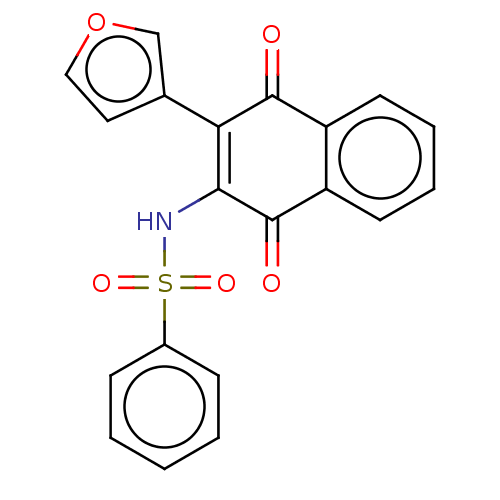
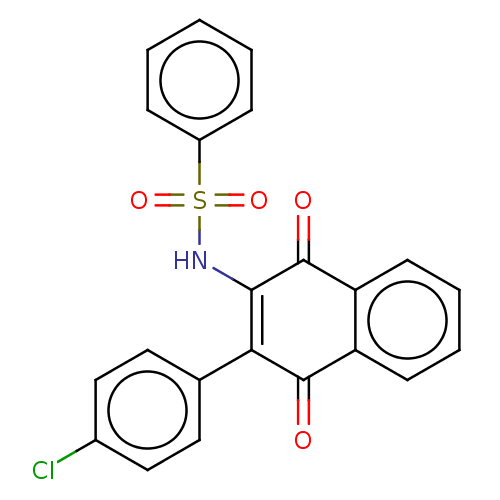
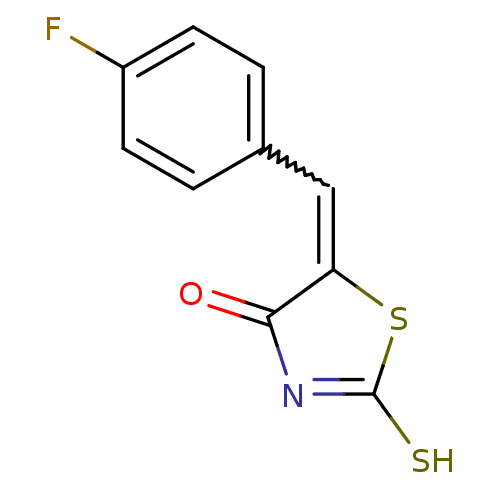
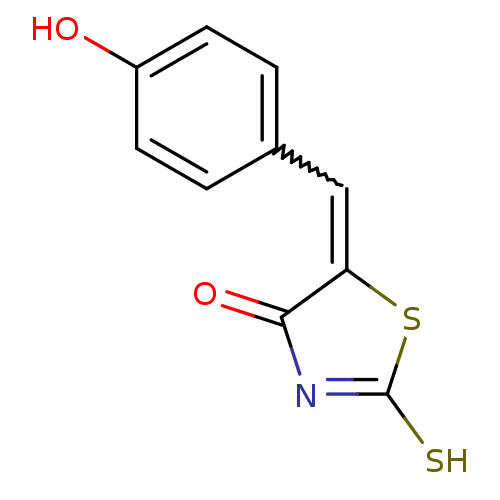
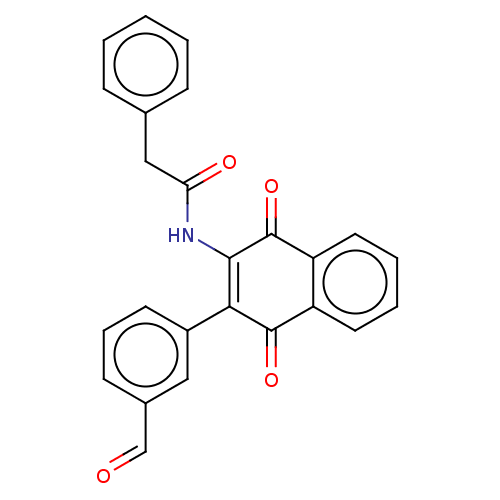
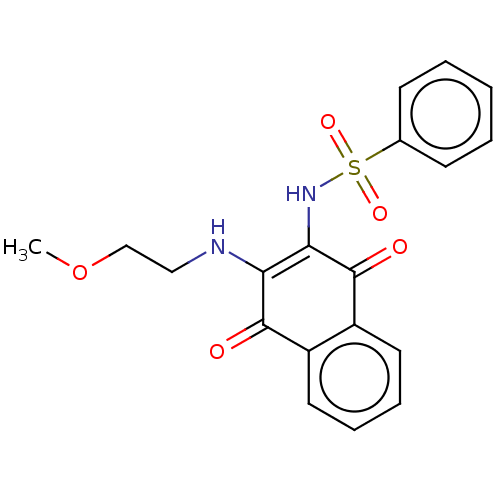
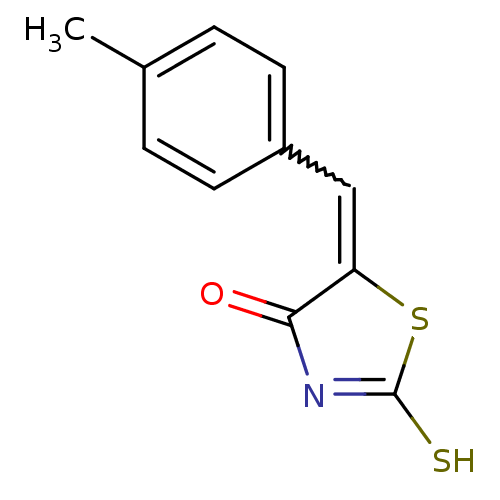
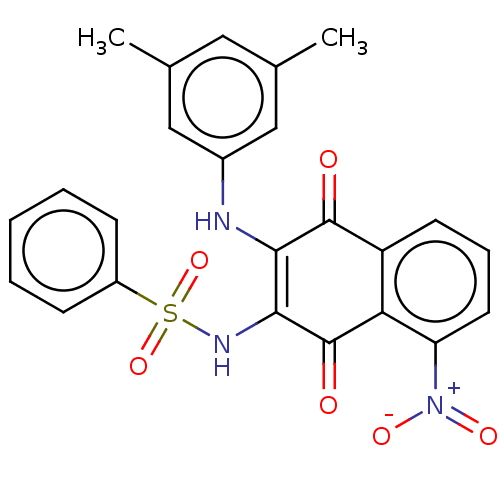
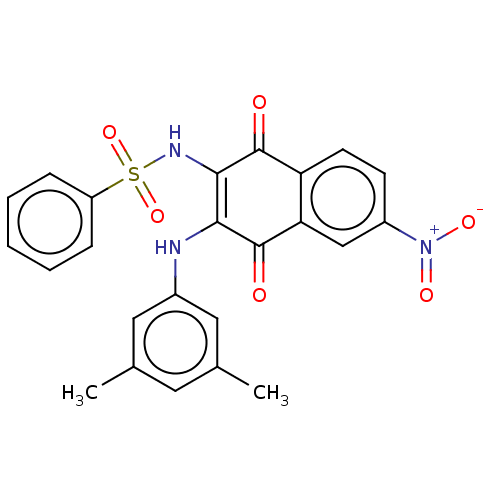
Affinity DataIC50: 120nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 138nMAssay Description:Inhibition of NAT1 in human ZR75 cell lysate assessed as acetylation of aryl amine using PABA as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 330nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 540nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of Homo sapiens (human) arylamine N-acetyltransferase 1More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.80E+3nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.10E+3nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.40E+3nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9.60E+3nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.21E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.21E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.29E+4nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.45E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.69E+4nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.76E+4nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.93E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.21E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's methodMore data for this Ligand-Target Pair