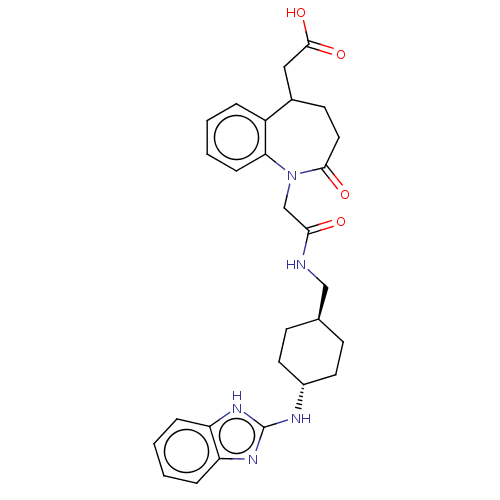
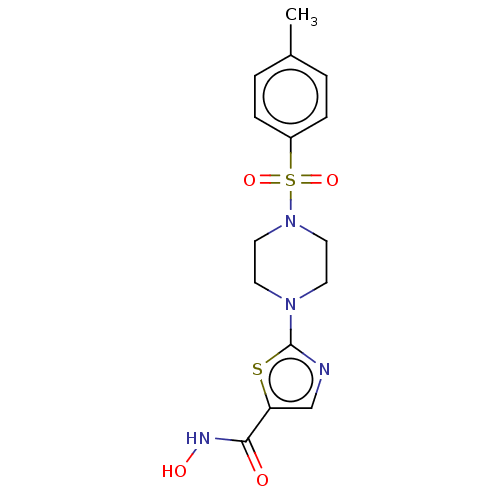
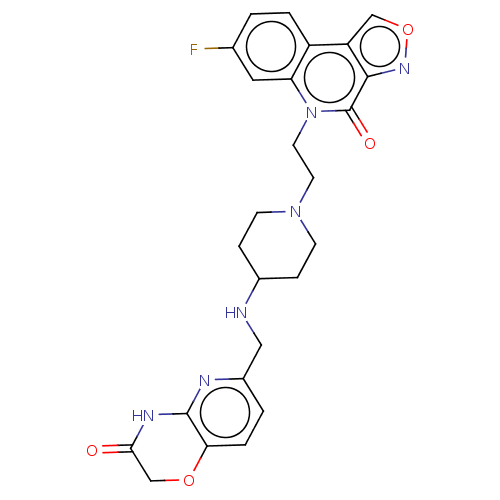
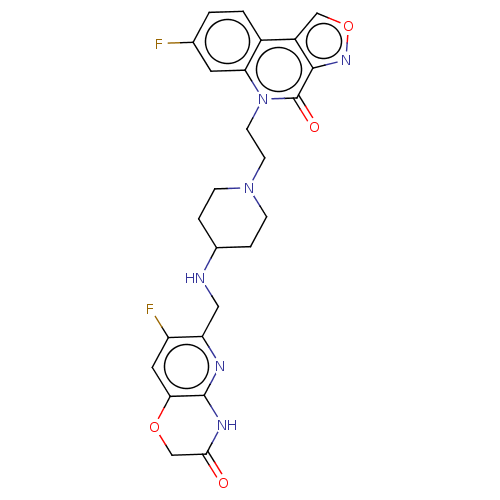
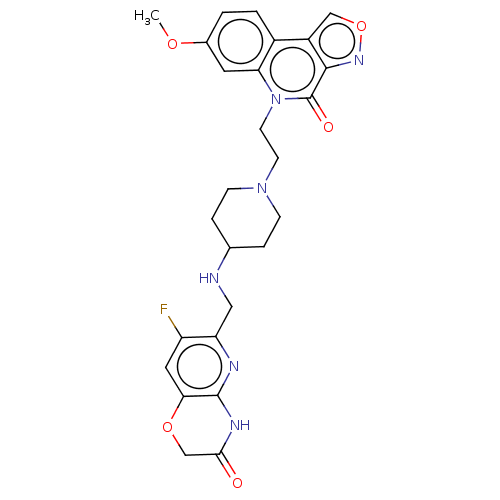
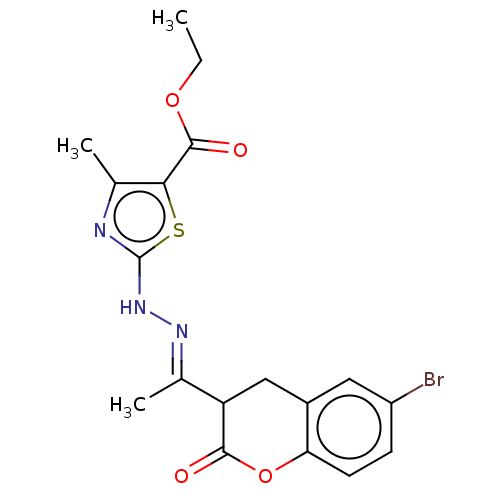
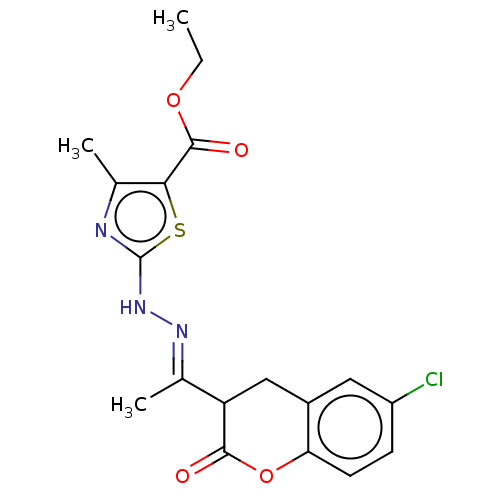
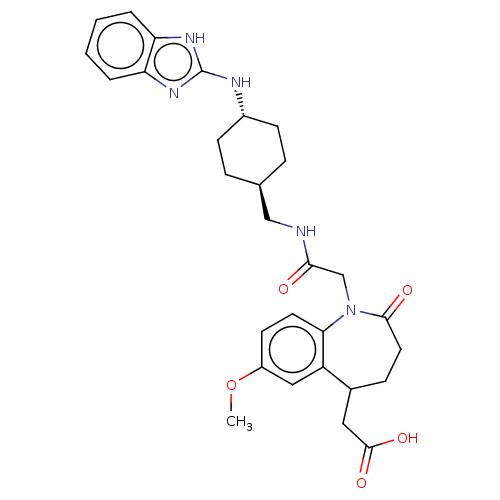
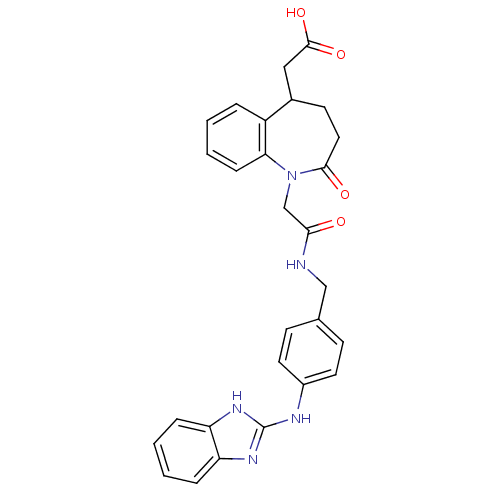
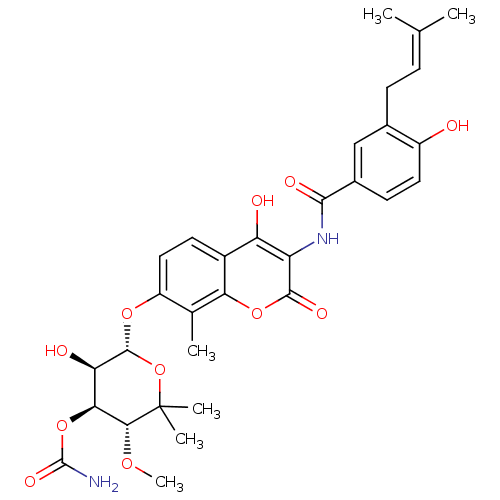
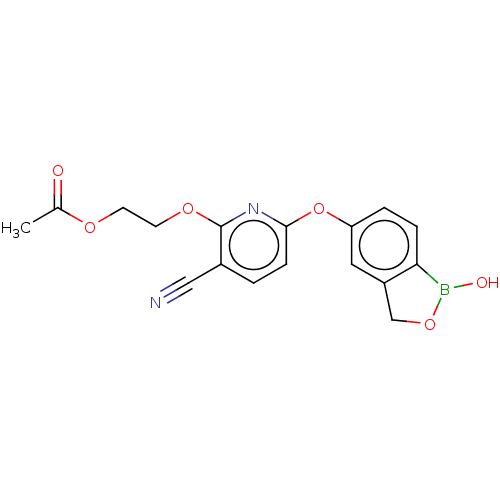
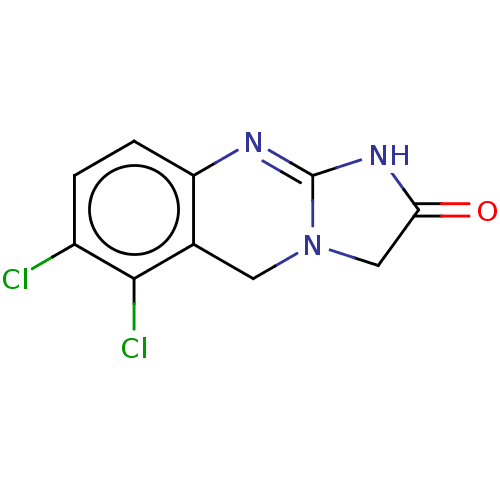
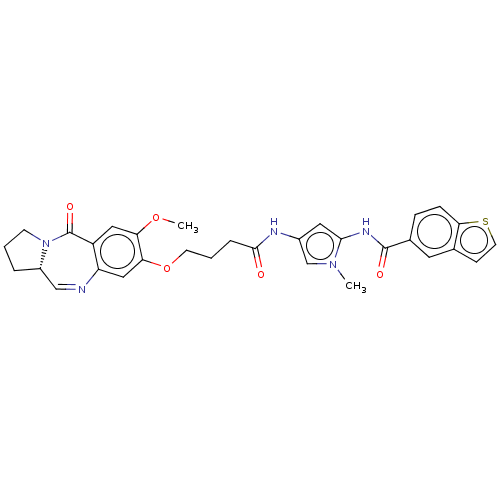
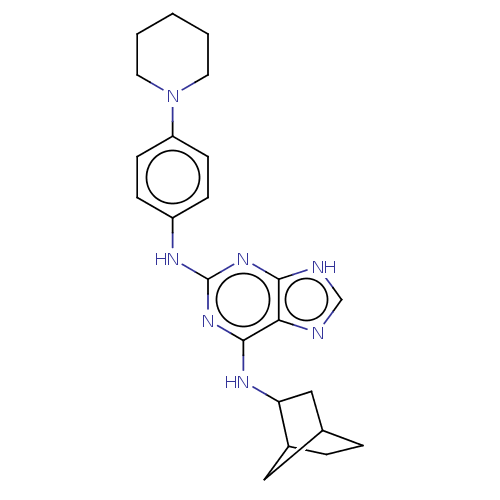
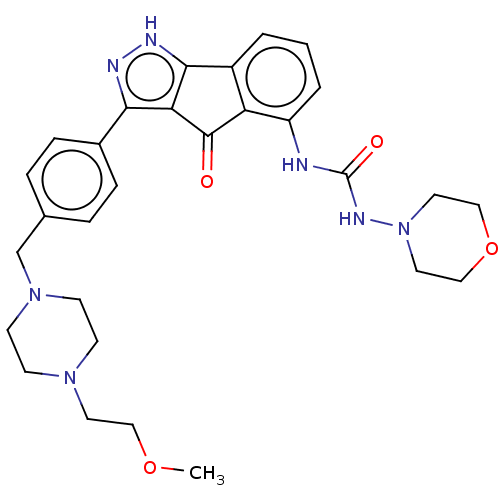
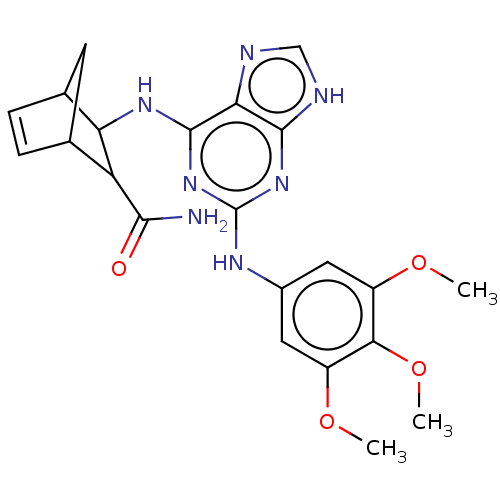
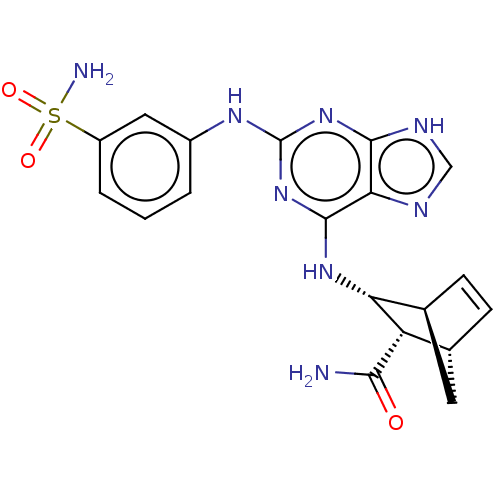
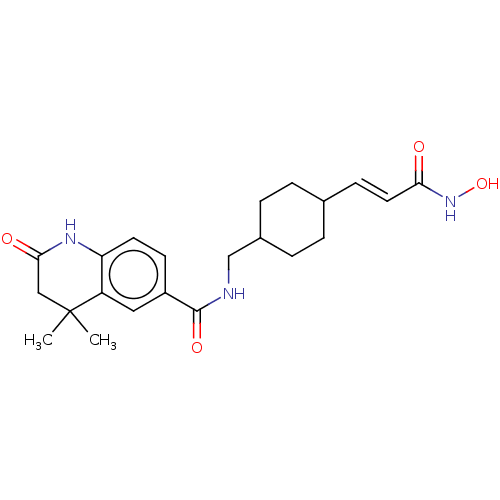
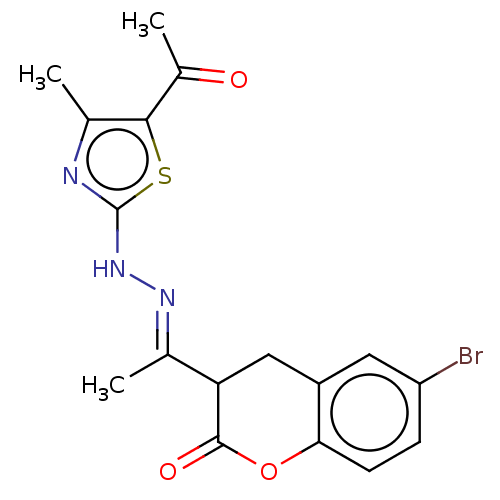
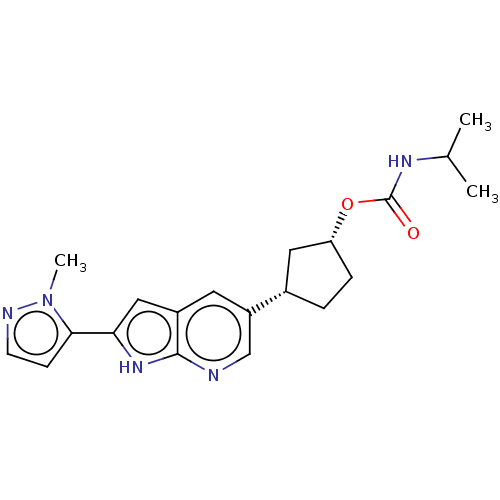
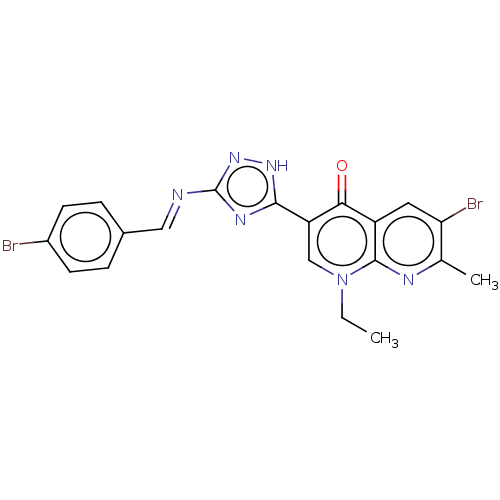
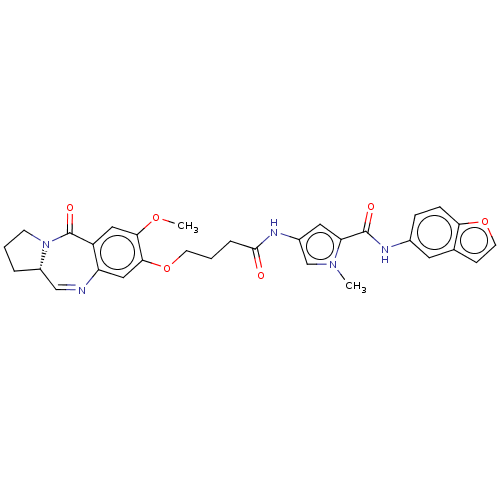
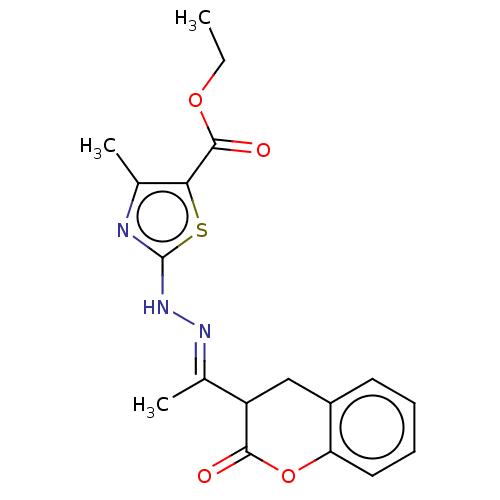
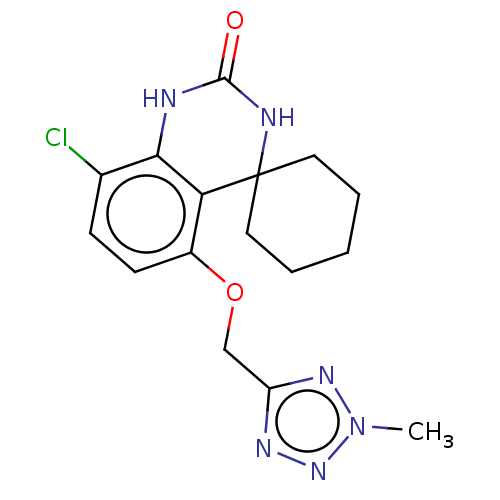
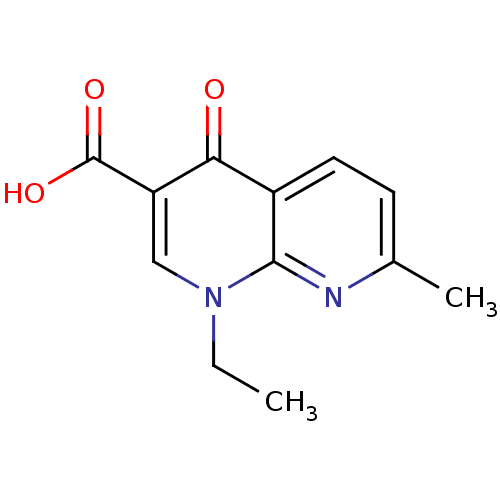
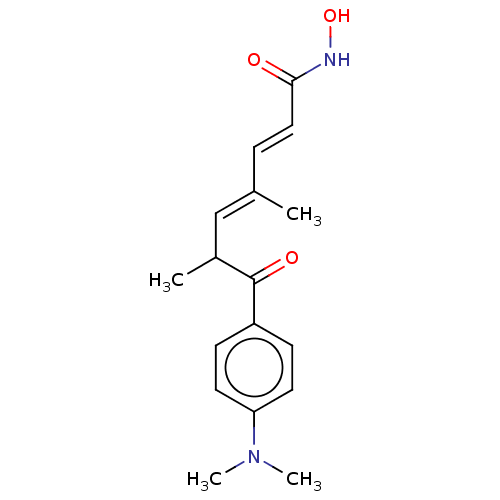
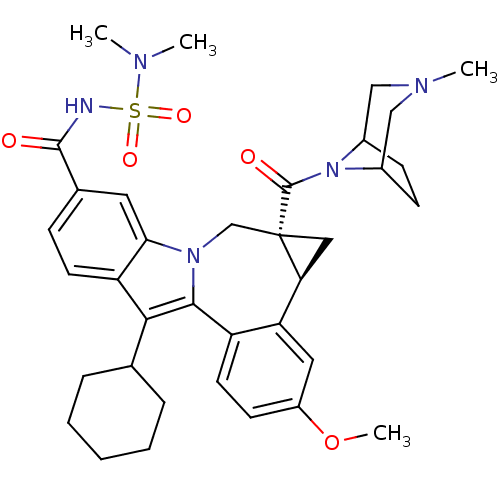
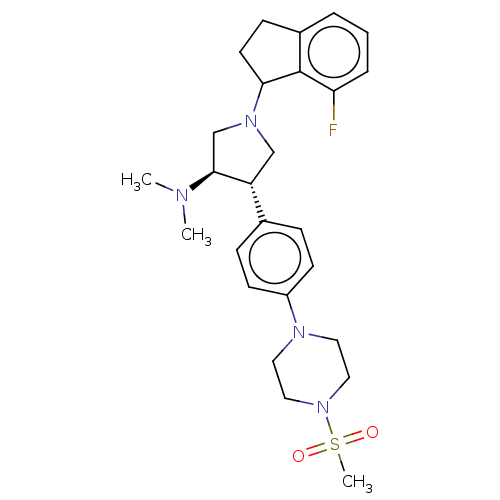
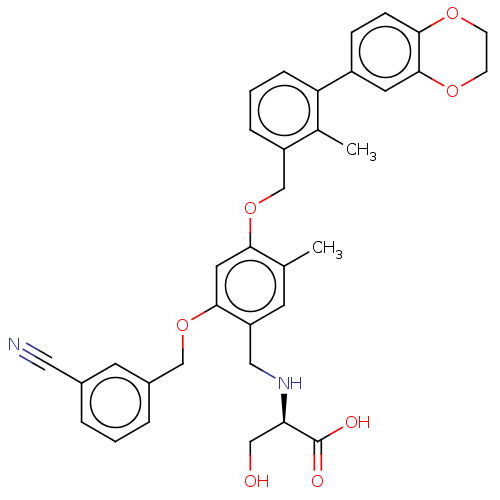
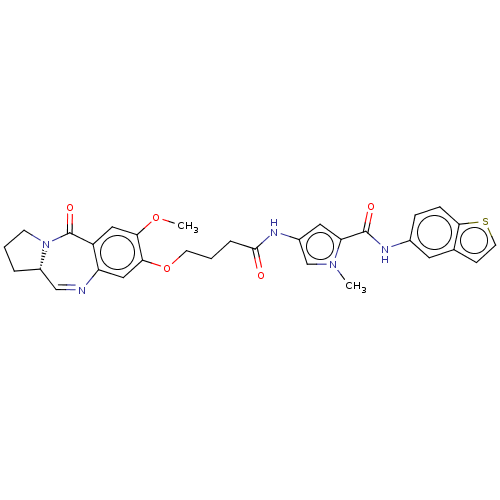
Affinity DataIC50: 0.0400nMAssay Description:Antagonist activity at human P2X7 receptor transfected in THP1 cells assessed as inhibition of benzoyl-ATP-induced changes in plasma membrane pore fo...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
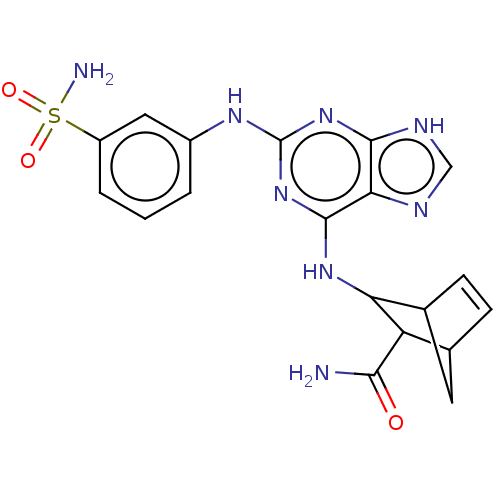
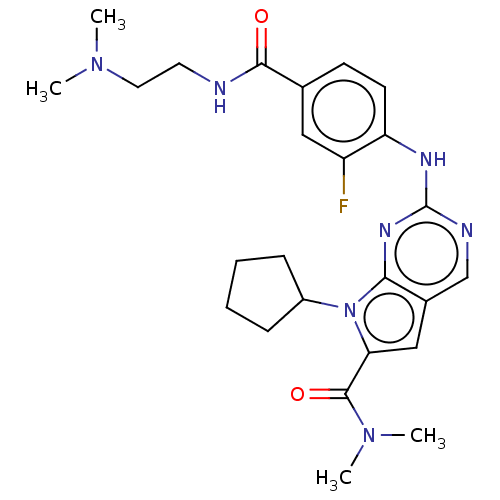
Affinity DataIC50: 0.260nMAssay Description:Antagonist activity at human P2X7 receptor transfected in THP1 cells assessed as inhibition of benzoyl-ATP-induced changes in plasma membrane pore fo...More data for this Ligand-Target Pair
In DepthDetails
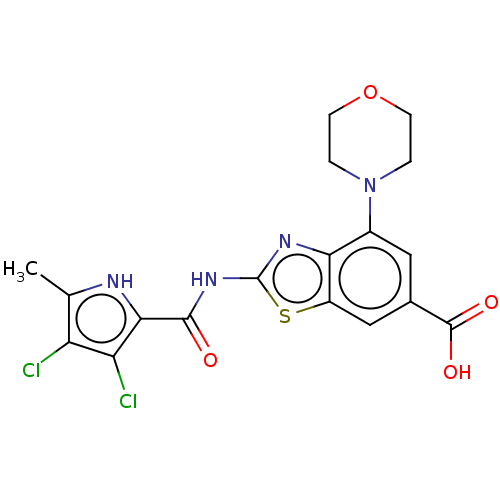
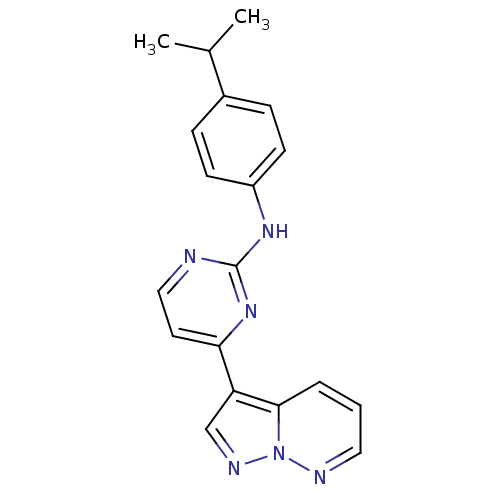
Affinity DataIC50: 0.5nMAssay Description:Antagonist activity at human P2X7 receptor transfected in THP1 cells assessed as inhibition of benzoyl-ATP-induced changes in plasma membrane pore fo...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
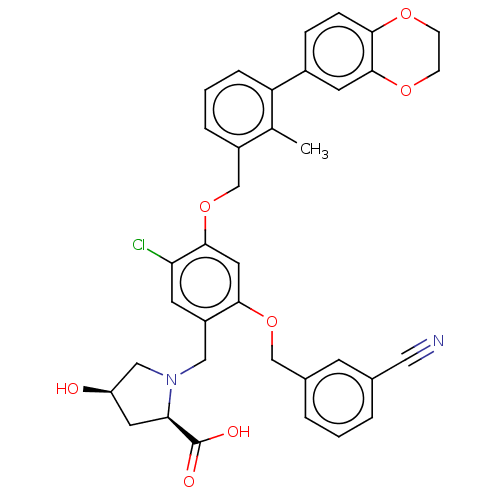
Affinity DataIC50: 0.700nMAssay Description:Activity at Histamine H2 receptor using functional H2 receptor assay on guinea pig atriumMore data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
Affinity DataIC50: 1nMAssay Description:Ability to displace [3H]glycine from strychnine-insensitive glycine receptorMore data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
Affinity DataIC50: 1.70nMAssay Description:Antagonist activity at human P2X7 receptor transfected in THP1 cells assessed as inhibition of benzoyl-ATP-induced changes in plasma membrane pore fo...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
Affinity DataIC50: 1.80nMAssay Description:Inhibitory activity against Dihydrofolate reductase in Neisseria gonorrhoeaeMore data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
Affinity DataIC50: 1.80nMAssay Description:Dissociation constant against VEGF (vascular endothelial growth factor) was determined.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 2nMAssay Description:Blocking activity against Beta-1 adrenergic receptor in spontaneously beating guinea pig atrial preparationsMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 2nMAssay Description:Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
Affinity DataIC50: 2nMAssay Description:Inhibition of Platelet-derived growth factor receptor in P19 cellsMore data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
In DepthDetails
Affinity DataIC50: 2.5nMAssay Description:In vitro antagonism of the 5-HT-3 receptor determined by inhibition of 5-HT-induced depolarization of the isolated rat vagus nerve.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 2.70nMAssay Description:The compound was evaluated for its relative binding affinity against mutant N131A scytalone dehydratase; Relative KiMore data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
In DepthDetails