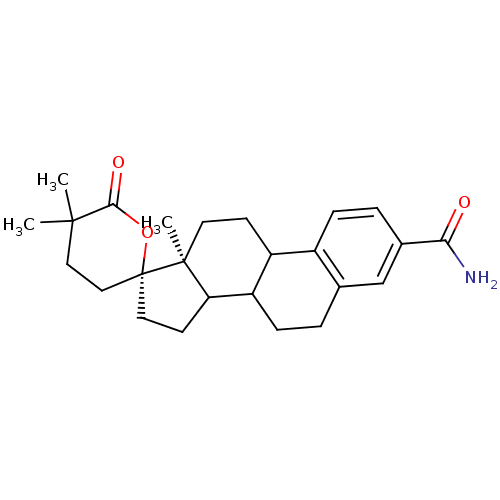
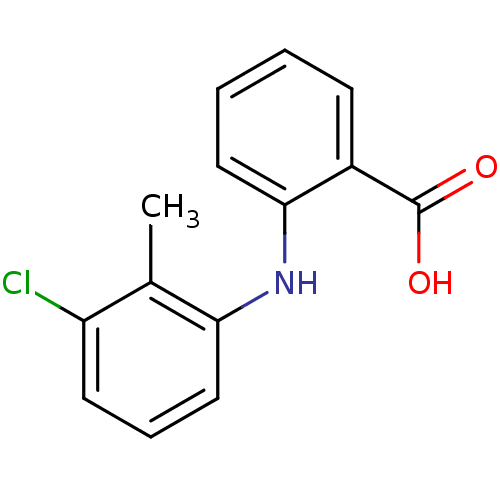
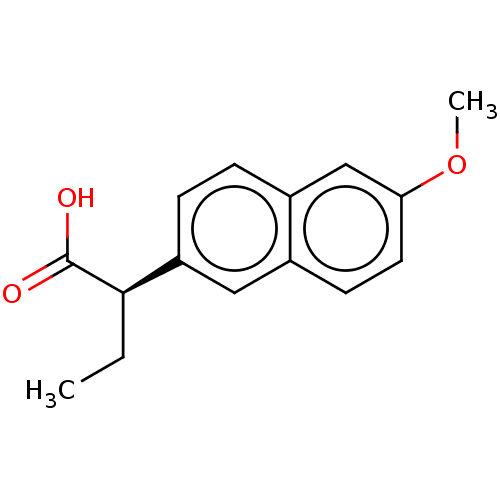
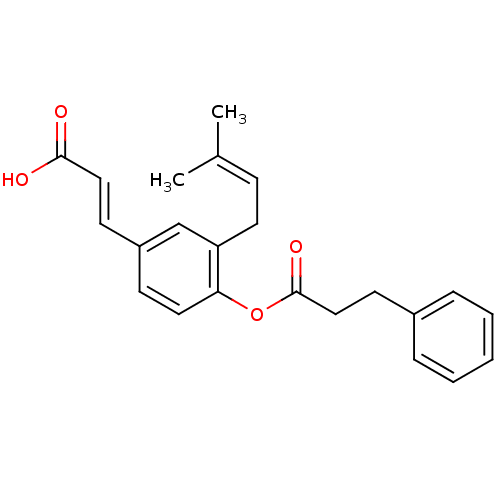
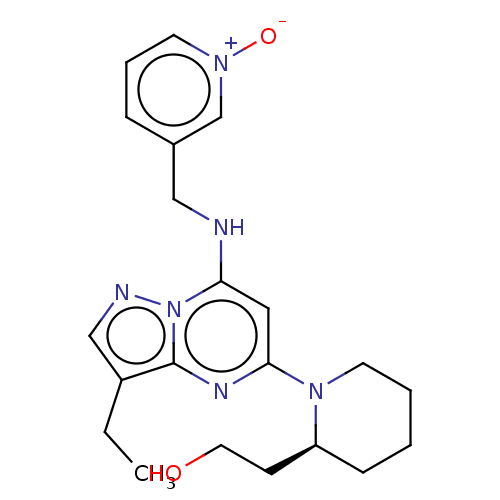
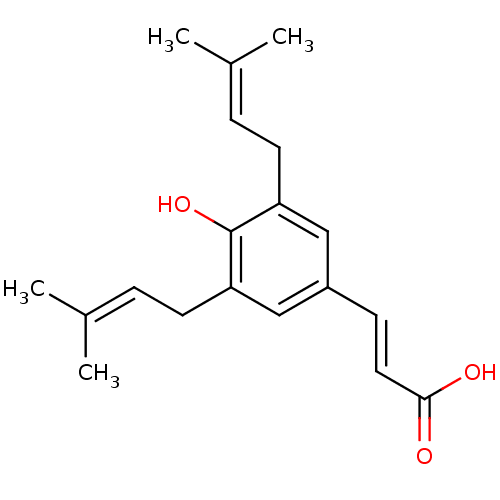
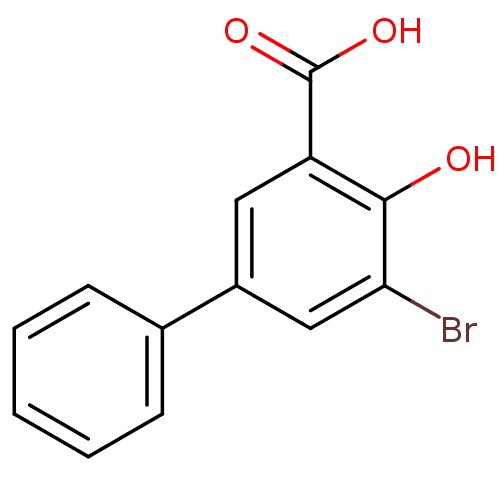
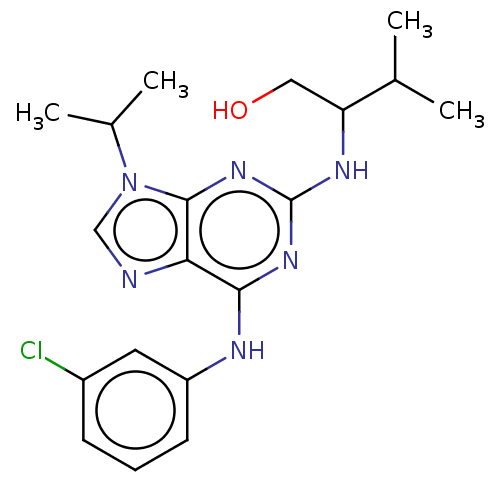
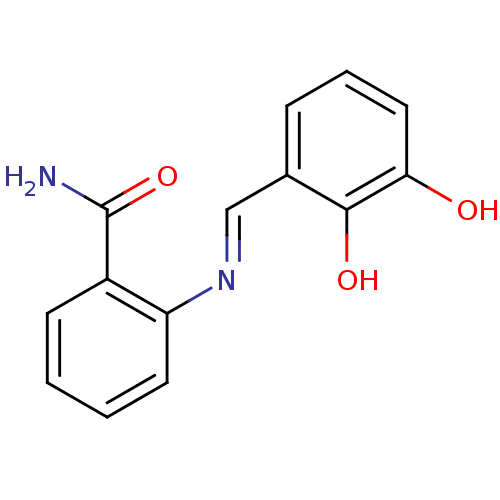
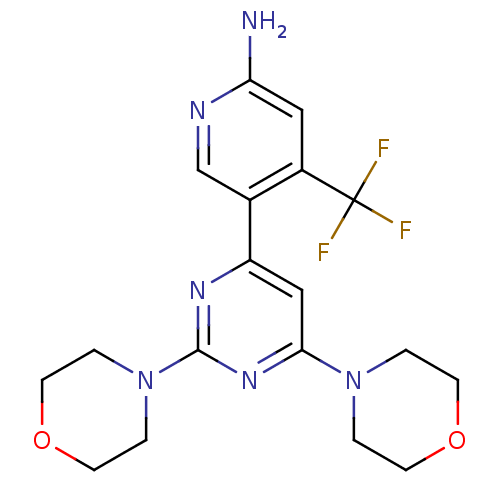
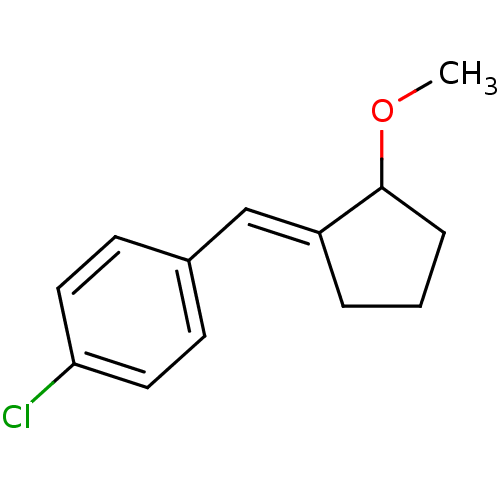
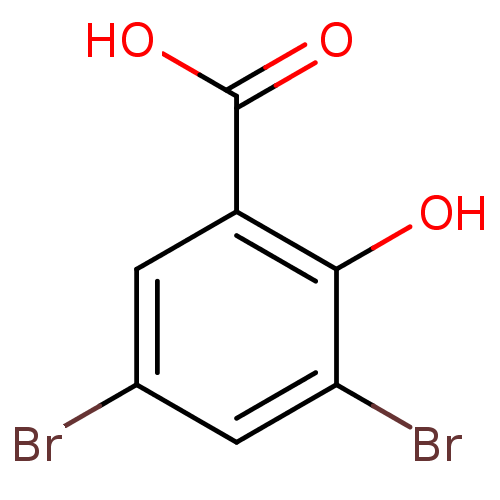
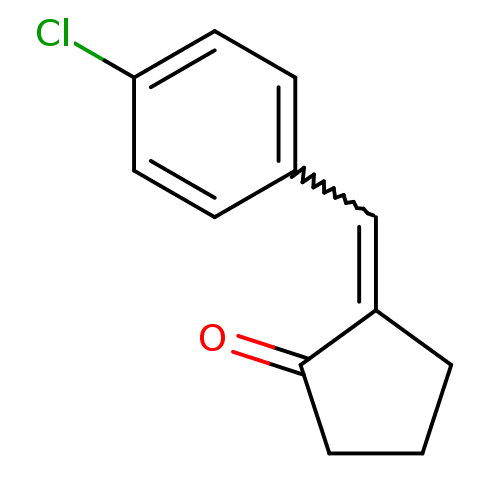
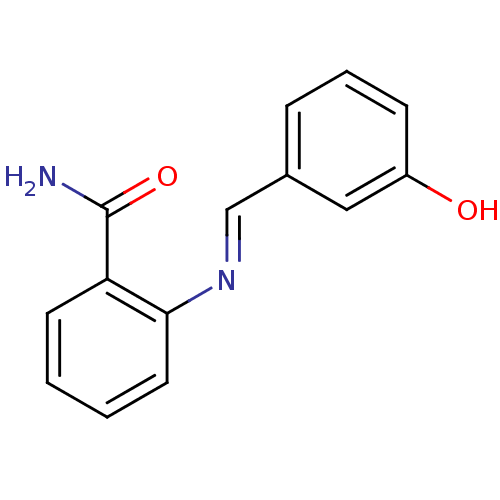
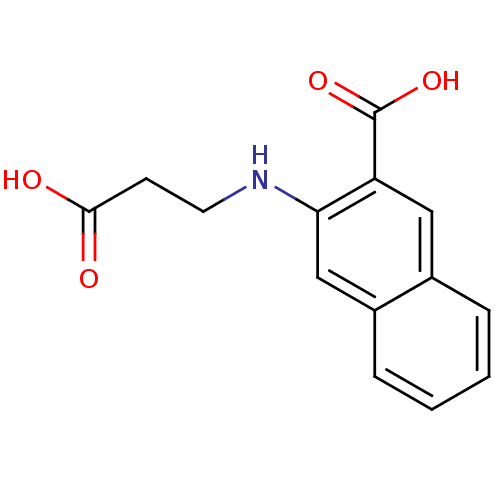
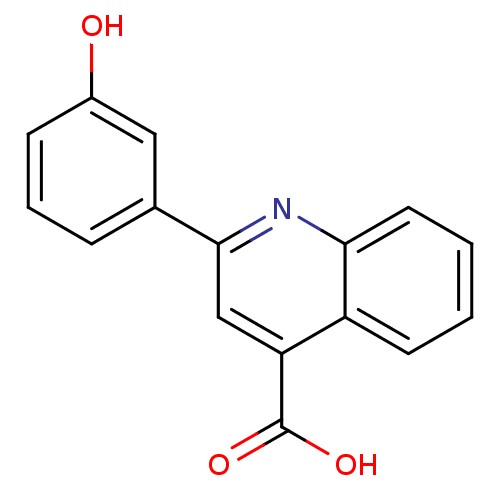
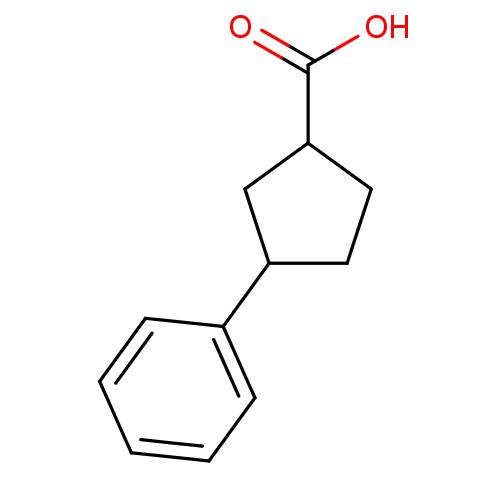
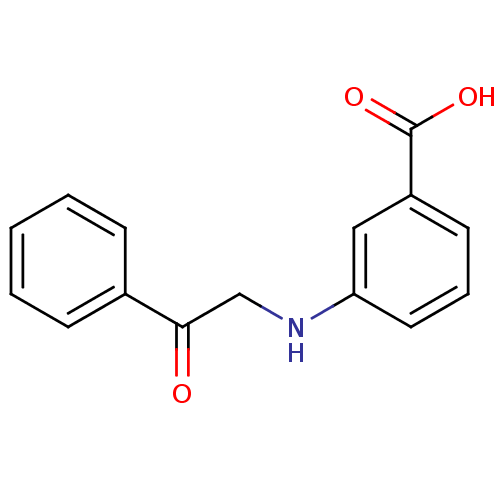
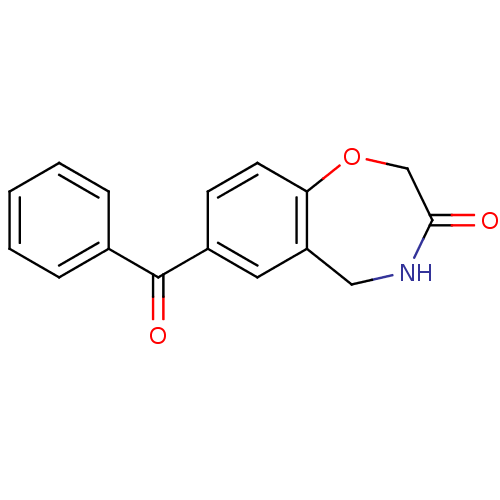
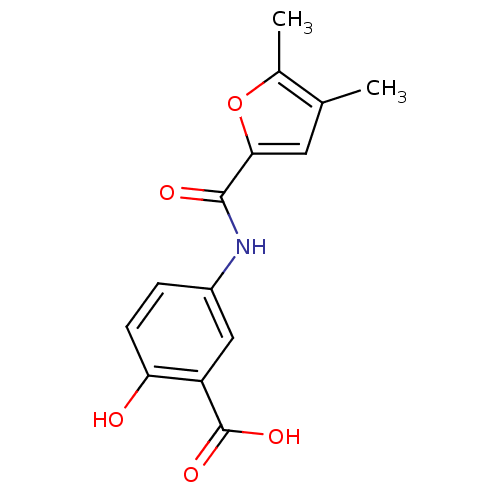
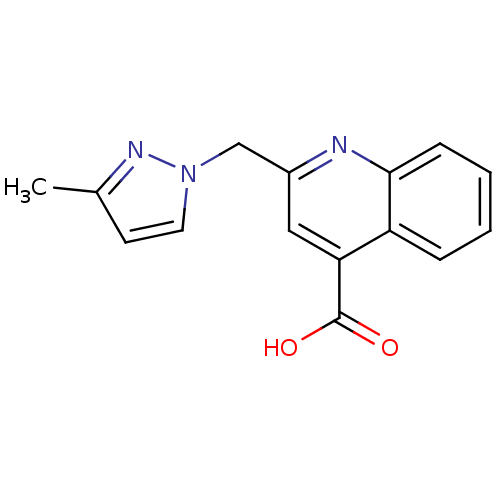
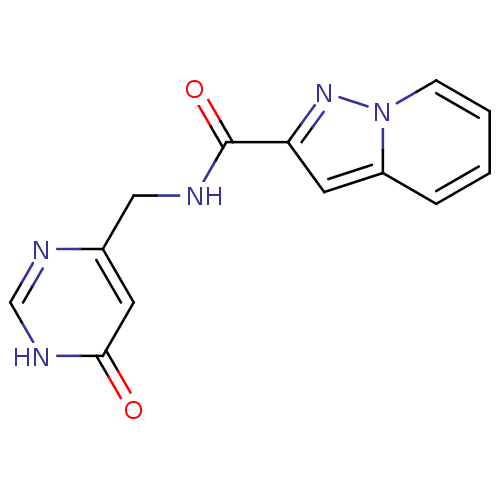
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 6.90nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
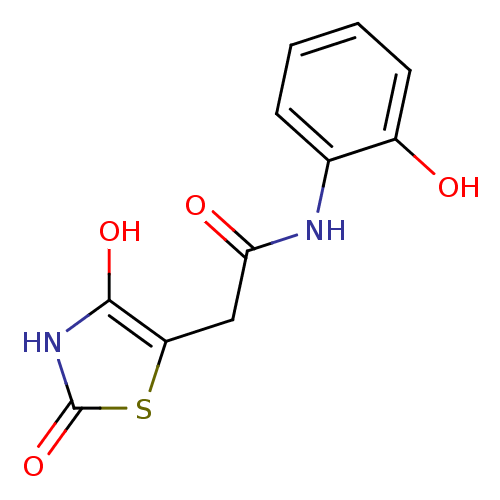
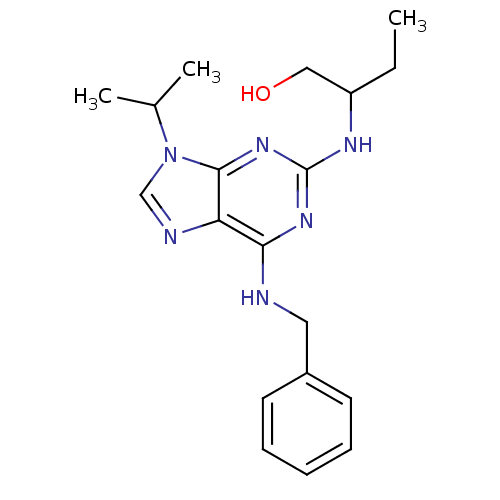
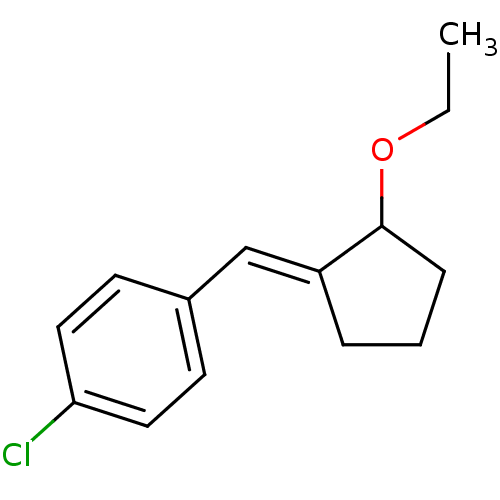
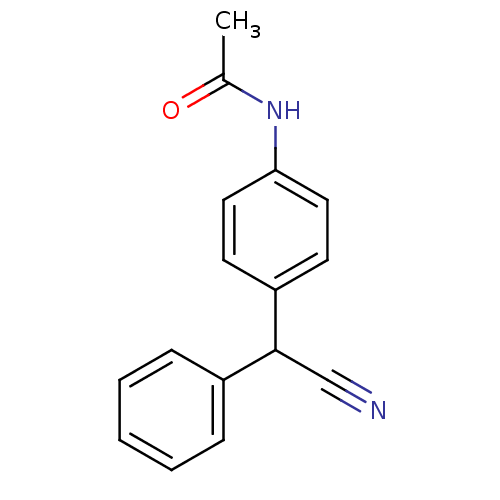
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 6.90nMAssay Description:Inhibition of human GST-tagged 17betaHSD5 expressed in Escherichia coli by radiometric assayMore data for this Ligand-Target Pair
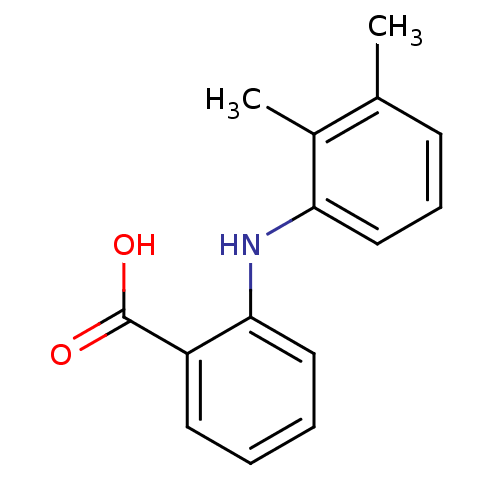
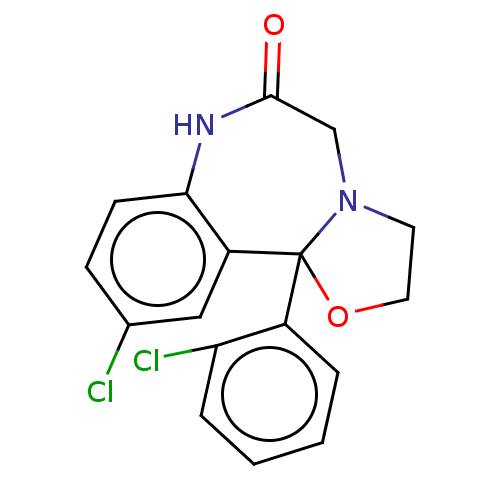
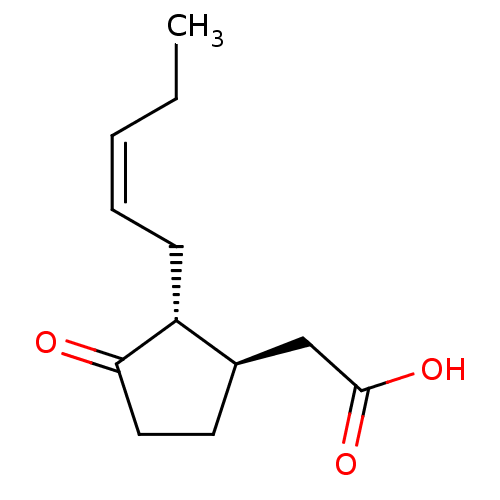
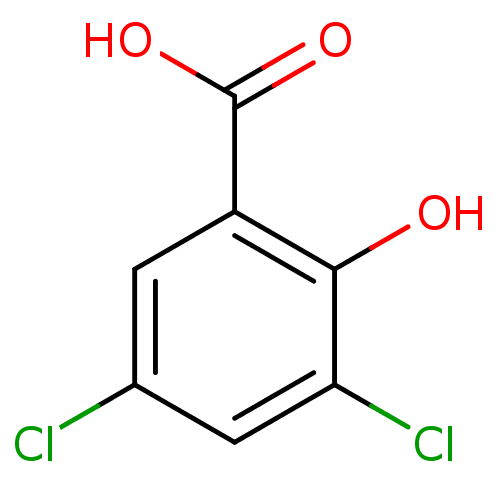
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 6.90nM ΔG°: -11.6kcal/molepH: 7.5 T: 2°CAssay Description:A radioactive assay was used for the enzyme kinetics in the presence of EM1404 at different concentrations for its Ki determination. Reactions were i...More data for this Ligand-Target Pair
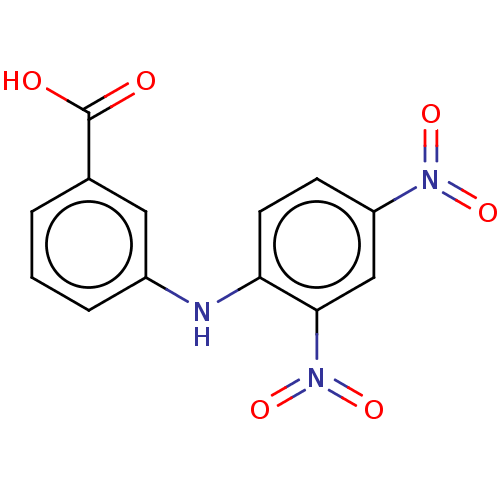
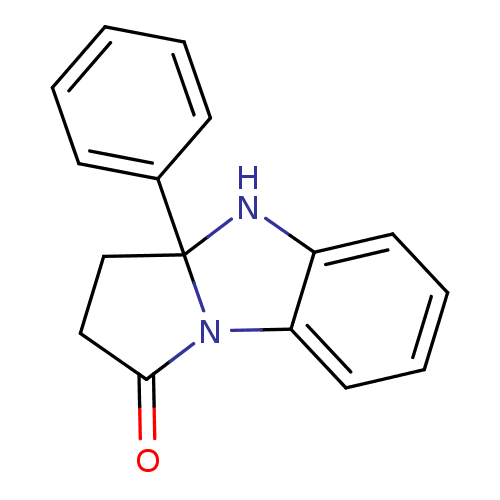
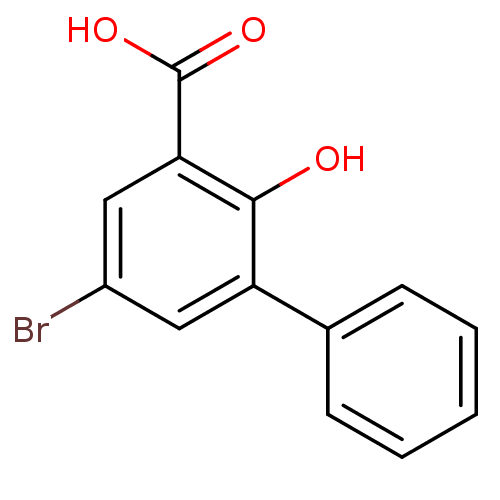
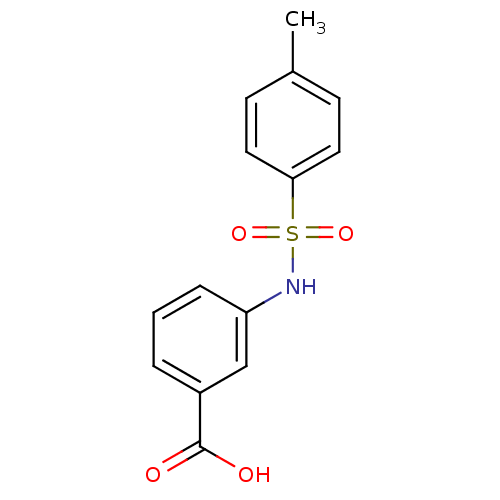
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 8nMAssay Description:Inhibition of human AKR1C3 using S-(+)-1,2,3,4-tetrahydro-1-naphthol as substrateMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 31nMAssay Description:Competitive inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preinc...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 56nMAssay Description:Competitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 56nMAssay Description:Competitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using NADP+ linked S-tetralol as substrate by fluorometr...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 56nMAssay Description:Noncompetitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry in prese...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 70nMAssay Description:Inhibition of human AKR1C3 expressed in Escherichia coli incubated for 30 mins in presence of NADPH regeneration system by UHPLC analysisMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 107nMAssay Description:Competitive inhibition of human recombinant AKR1C3 assessed as S-tetralol oxidation by Cheng-Prusoff equation analysisMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 140nM ΔG°: -9.50kcal/molepH: 7.0 T: 2°CAssay Description:A fluorescence assay was used to determine the kinetic constants for the oxidation of 3alpha-androstanediol. The fluorescence emission of NADPH at 45...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 270nM ΔG°: -9.10kcal/molepH: 7.0 T: 2°CAssay Description:A fluorescence assay was used to determine the kinetic constants for the oxidation of 3alpha-androstanediol. The fluorescence emission of NADPH at 45...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 300nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 380nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 680nMAssay Description:Noncompetitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry in prese...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 680nMAssay Description:Competitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 750nMAssay Description:Competitive inhibition of human recombinant AKR1C3 using assessed as reduction in NADPH-dependent reduction of delat4-androsten-3,17-dione preincubat...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of human AKR1C3 expressed in Escherichia coli incubated for 30 mins in presence of NADPH regeneration system by UHPLC analysisMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.50E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.10E+3nM ΔG°: -7.87kcal/molepH: 7.0 T: 2°CAssay Description:Enzyme activity was measured in the reductive direction against varying concentrations of androsterone. Initial velocities were measured by observing...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.73E+3nMAssay Description:Competitive inhibition of human recombinant AKR1C3 assessed as S-tetralol oxidation by Cheng-Prusoff equation analysisMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 3.10E+3nM ΔG°: -7.64kcal/molepH: 7.0 T: 2°CAssay Description:Enzyme activity was measured in the reductive direction against varying concentrations of androsterone. Initial velocities were measured by observing...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 4.20E+3nMAssay Description:Inhibition of human recombinant type 2 3-alpha-HSD expressed in Escherichia coli JM109More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 5.50E+3nMAssay Description:Inhibition of human AKR1C3 expressed in Escherichia coli incubated for 30 mins in presence of NADPH regeneration system by UHPLC analysisMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 6.00E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 8.20E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.20E+4nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.40E+4nMAssay Description:Inhibition of human AKR1C3 expressed in Escherichia coli incubated for 30 mins in presence of NADPH regeneration system by UHPLC method based Linewea...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.62E+4nMAssay Description:Inhibition of human recombinant GST-tagged AKR1C3 expressed in Escherichia coli BL21More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.05E+4nMAssay Description:Inhibition of human recombinant GST-tagged AKR1C3 expressed in Escherichia coli BL21More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.10E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.10E+4nMAssay Description:Inhibition of human recombinant type 2 3-alpha-HSD expressed in Escherichia coli JM109More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.30E+4nMAssay Description:Inhibition of human recombinant type 2 3-alpha-HSD expressed in Escherichia coli JM109More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.40E+4nMAssay Description:Inhibition of recombinant full-length human N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21 (DE3) using phenanthrenequinone as subst...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 3.20E+4nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 3.34E+4nMAssay Description:Inhibition of human recombinant GST-tagged AKR1C3 expressed in Escherichia coli BL21More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 3.60E+4nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 4.40E+4nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 5.40E+4nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 6.96E+4nMAssay Description:Inhibition of human recombinant GST-tagged AKR1C3 expressed in Escherichia coli BL21More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 7.20E+4nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 7.60E+4nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 8.20E+4nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 8.70E+4nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 9.40E+4nM ΔG°: -5.49kcal/molepH: 7.4 T: 2°CAssay Description:The activity was assayed by measuring the rate of change in NADPH fluorescence (at 455 nm with an excitation wavelength of 340 nm) at 298 K. When the...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.11E+5nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.17E+5nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.18E+5nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair

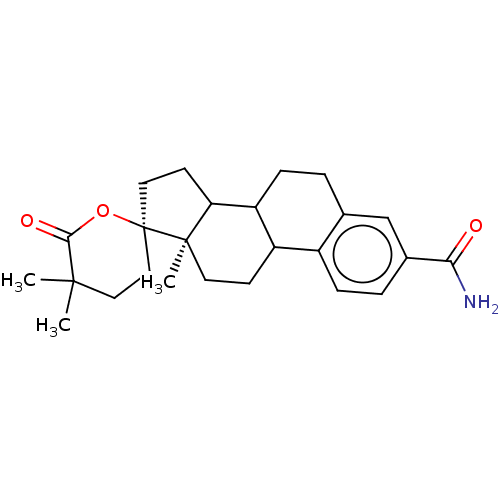
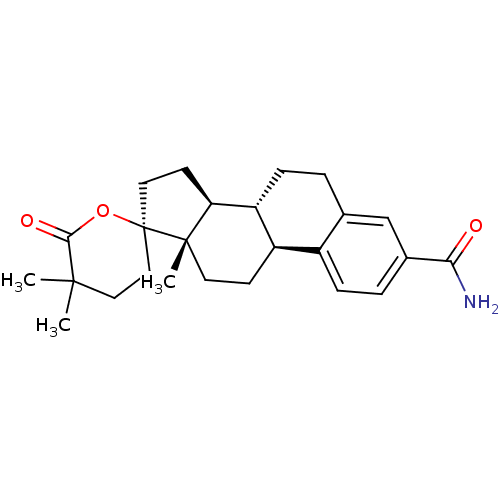
 3D Structure (crystal)
3D Structure (crystal)