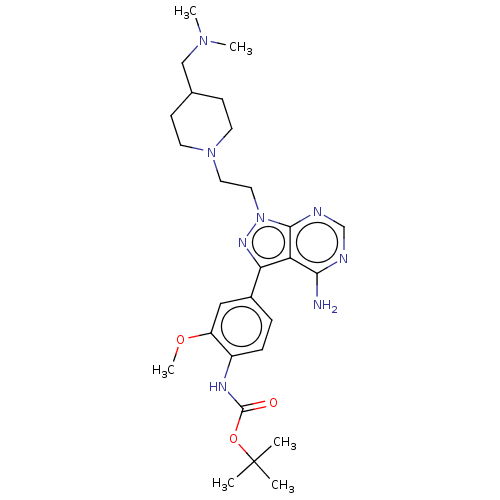
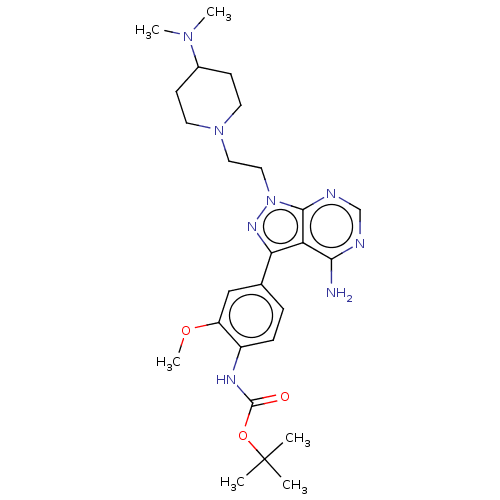
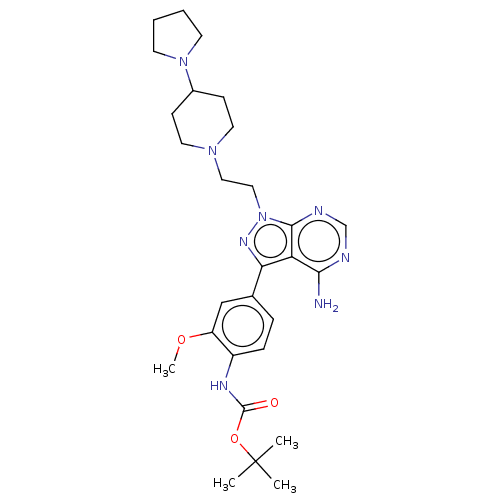
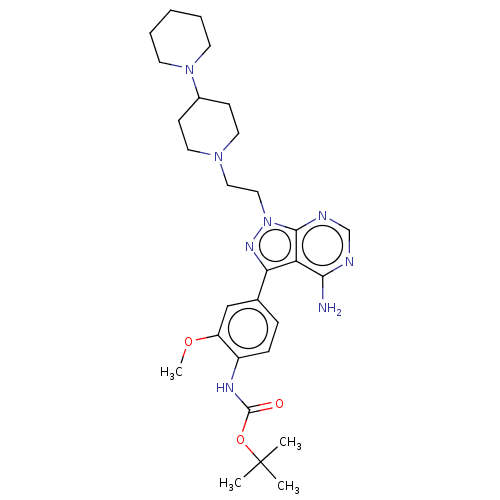
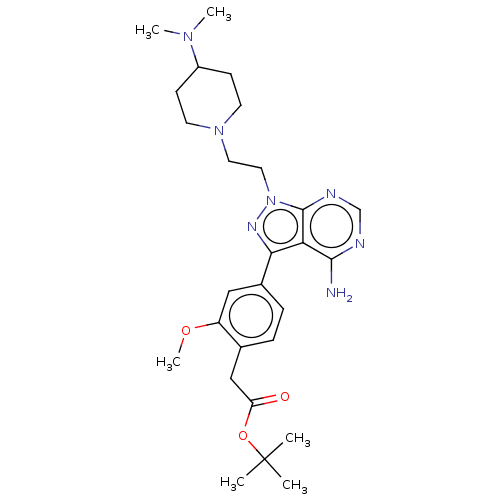
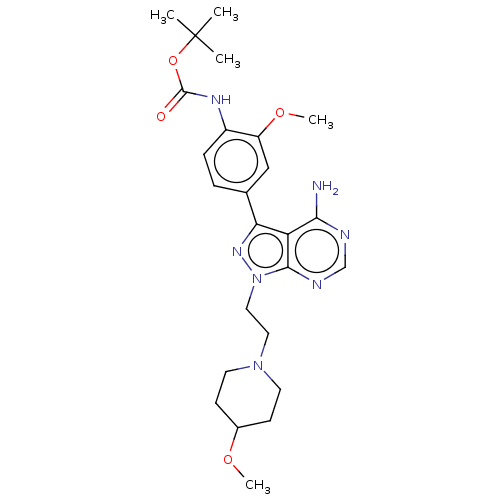
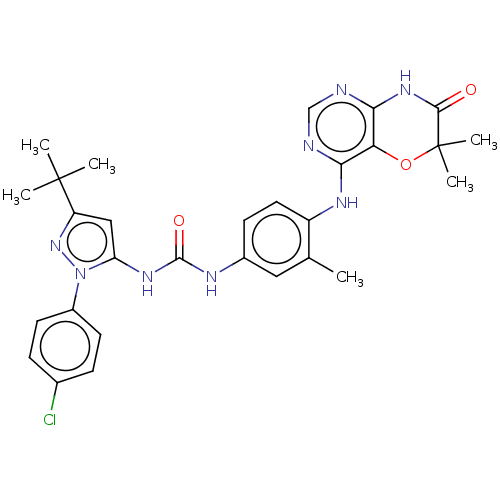
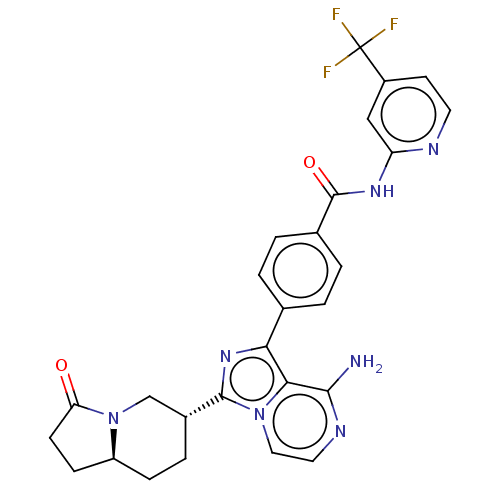
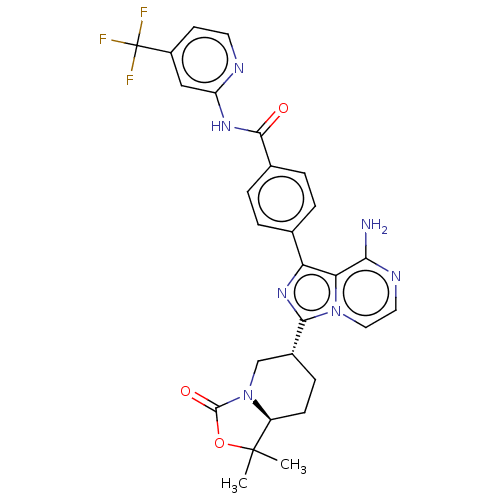
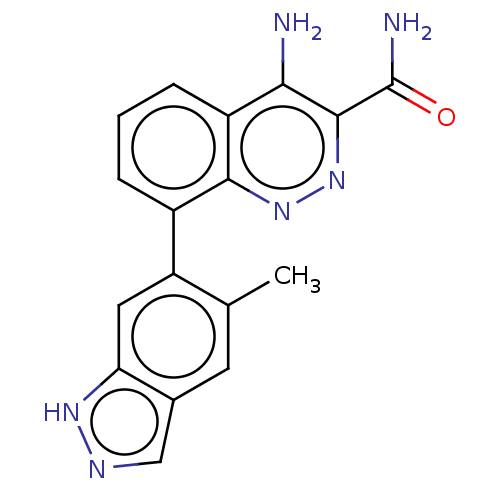
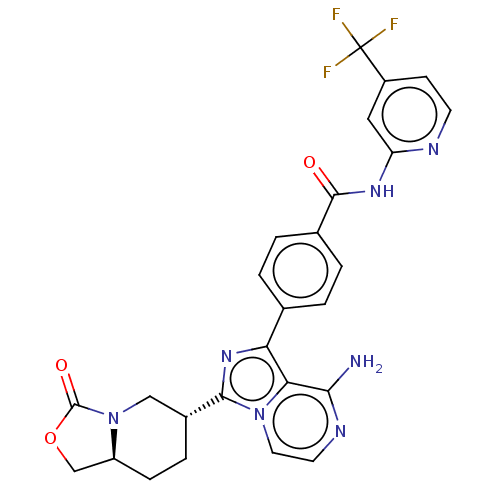
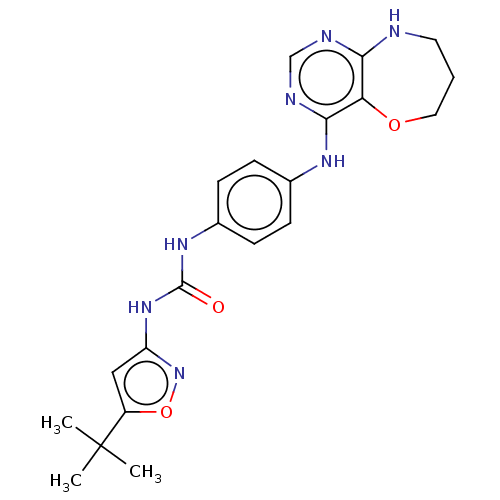
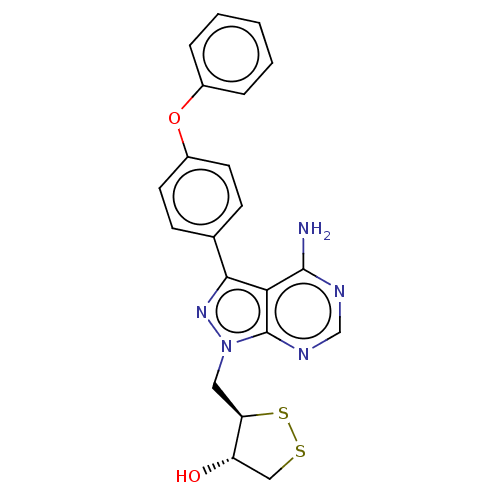
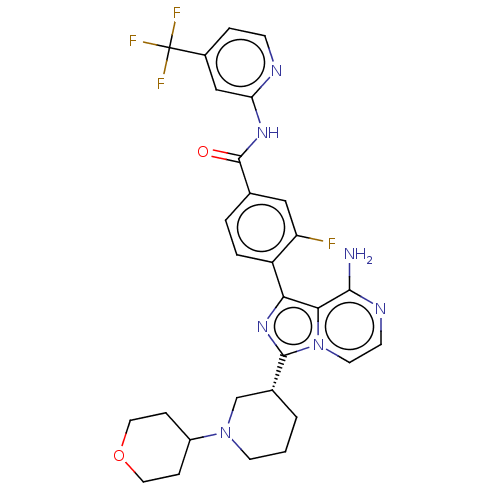
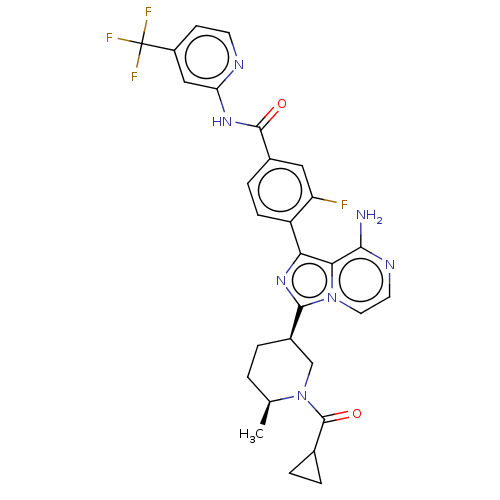
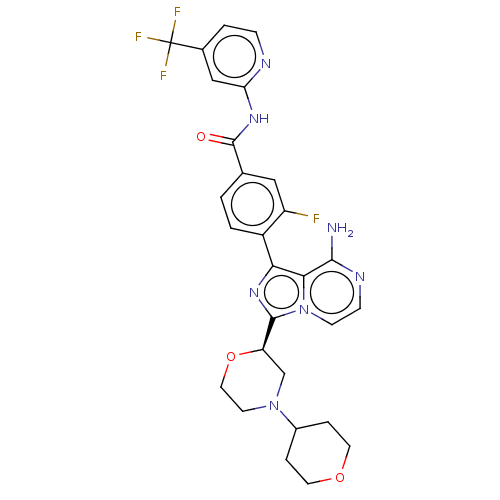
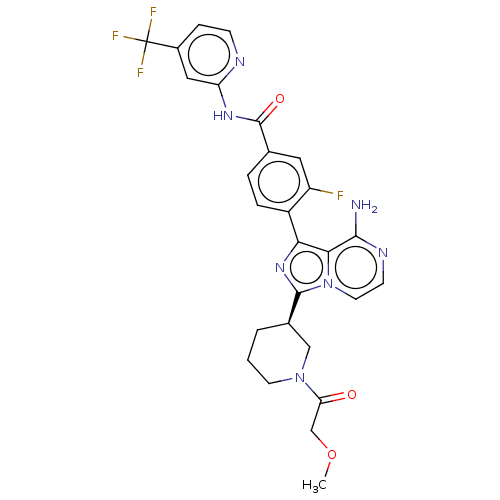
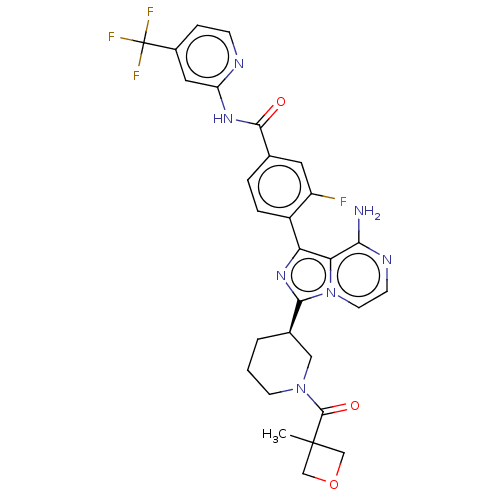
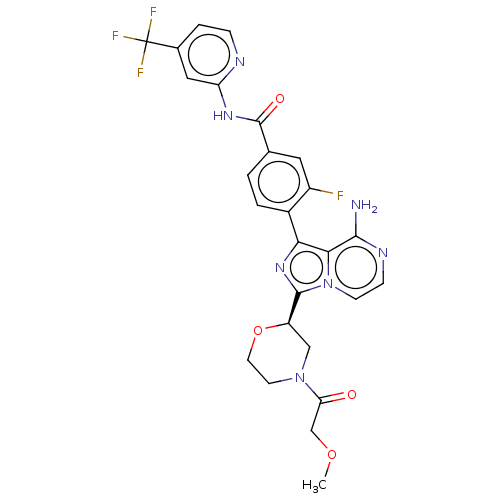
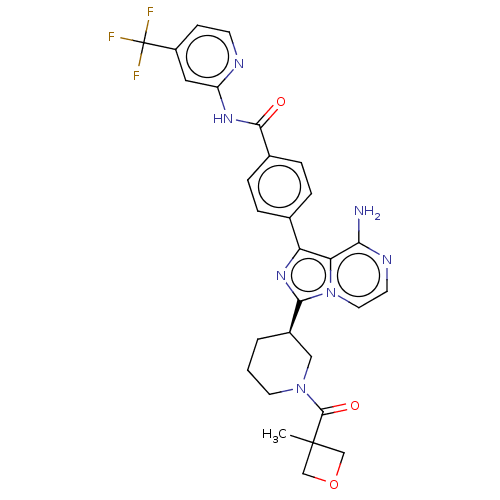
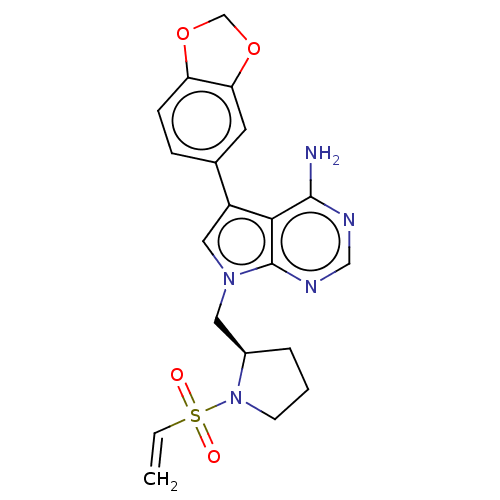
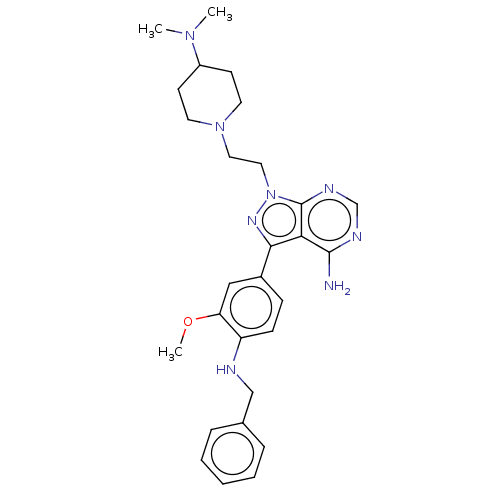
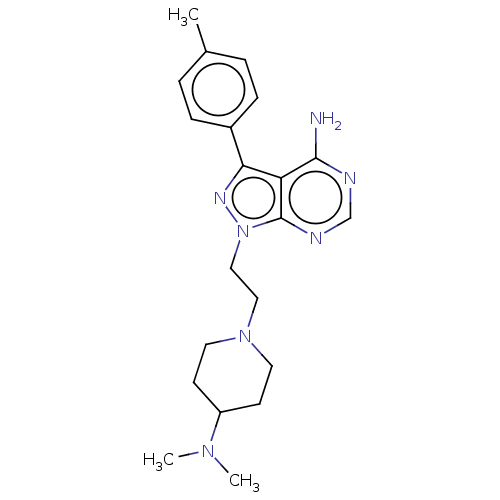
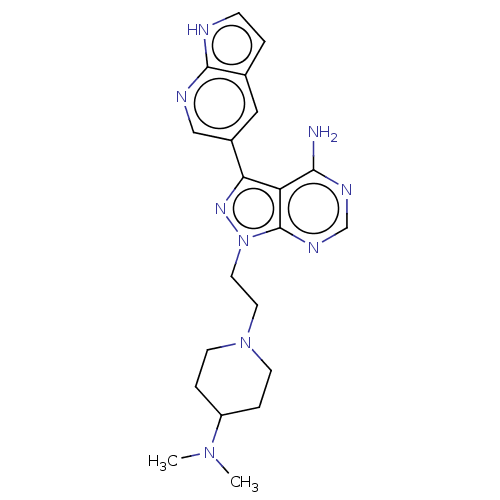
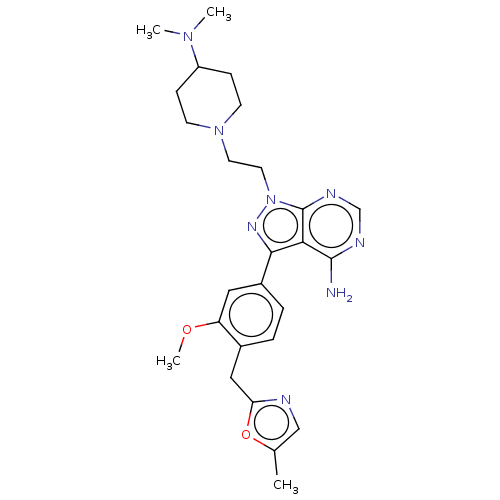
Affinity DataIC50: 1.30nMAssay Description:Binding affinity to human FRK using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assayMore data for this Ligand-Target Pair
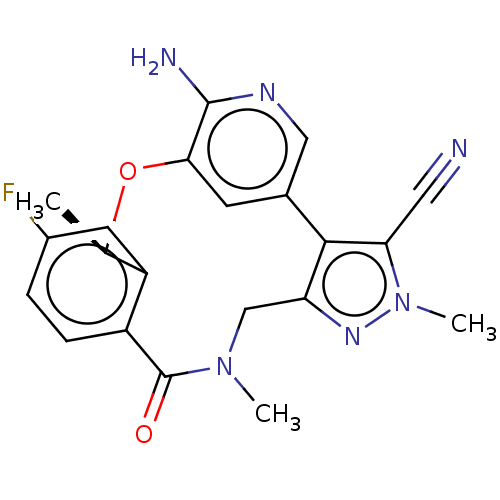
Affinity DataIC50: 4.60nMAssay Description:Inhibition of human FRK using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assayMore data for this Ligand-Target Pair
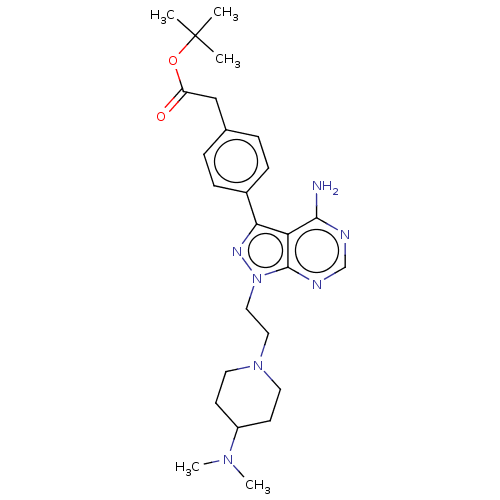
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
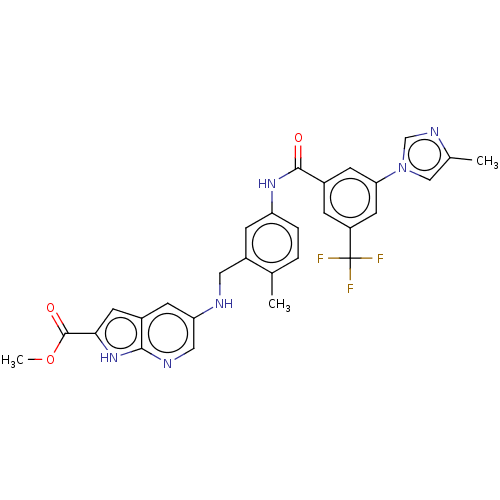
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of human FRK using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of human recombinant PTK5 (218 to end residues) using GGEEEEYFELVKKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibition of FRK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of human PTK5 in presence of ATP by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of FRK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibition of FRK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Inhibition of human recombinant FrkMore data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:Inhibition of FRK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of recombinant human PTK5 (218 to end residues) using GGEEEEYFELVKKKK as substrate incubated for 40 mins in presence of [gamma33P]ATP by s...More data for this Ligand-Target Pair
Affinity DataIC50: 52nMAssay Description:IC50 Table 13-20: The protocol calls for test compound of the invention to be incubated with kinase, substrate, cofactors, and radio-isotope-labeled ...More data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:Inhibition of FRK (unknown origin) by TR-FRET-based Z'-LYTE assayMore data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 62nMAssay Description:Inhibition of GST-tagged full length human recombinant FRK expressed in baculovirus using Fluorescein-Poly GT as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Inhibition of human FRK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m...More data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Inhibition of human FRK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m...More data for this Ligand-Target Pair
Affinity DataIC50: 85nMAssay Description:Inhibition of FRK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 111nMAssay Description:Inhibition of human FRKMore data for this Ligand-Target Pair
Affinity DataIC50: 132nMAssay Description:Inhibition of FRK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 151nMAssay Description:Inhibition of FRK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 154nMAssay Description:Inhibition of FRK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 187nMAssay Description:Inhibition of human FRKMore data for this Ligand-Target Pair
Affinity DataIC50: 214nMAssay Description:Inhibition of FRK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 361nMAssay Description:Inhibition of human FRKMore data for this Ligand-Target Pair
Affinity DataIC50: 379nMAssay Description:Inhibition of human FRK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m...More data for this Ligand-Target Pair
Affinity DataIC50: 379nMAssay Description:Inhibition of human FRK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m...More data for this Ligand-Target Pair
Affinity DataIC50: 472nMAssay Description:Inhibition of FRK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged FRK (218 to end residues) expressed in baculovirus Sf21 insect cells preincubated for 5 mins f...More data for this Ligand-Target Pair
Affinity DataIC50: 530nMAssay Description:Inhibition of FRK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair