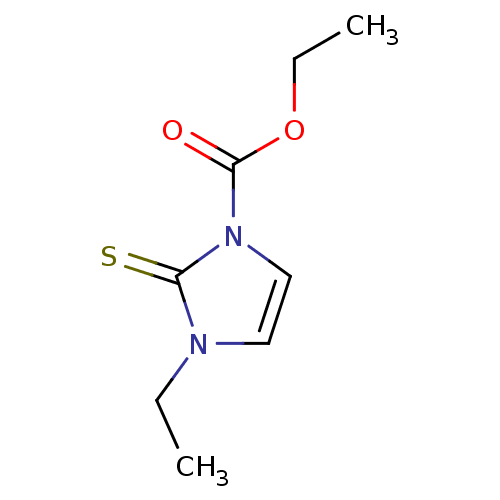
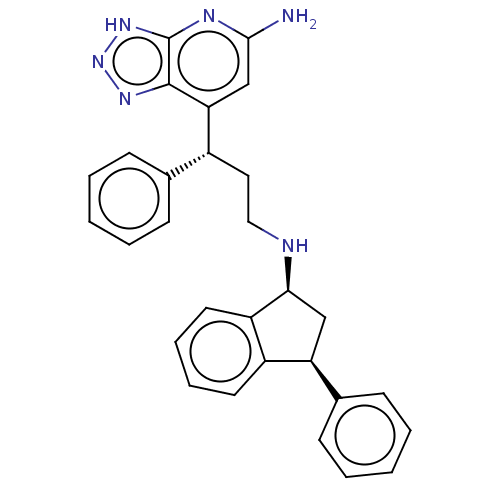
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
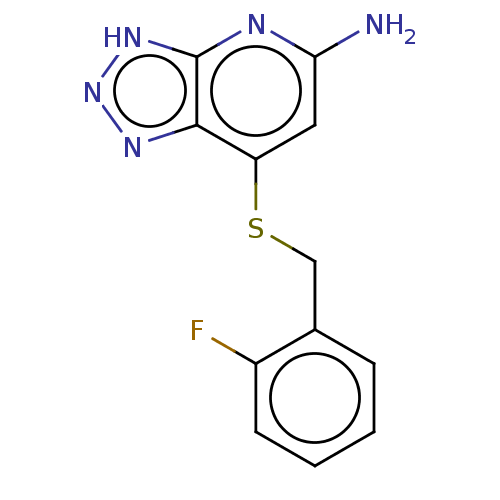
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of human LPO assessed as reduction in H2O2 catalyzed 3,5-iodo tyrosine formation from 3-iodotyrosine and potassium iodide preincubated for...More data for this Ligand-Target Pair
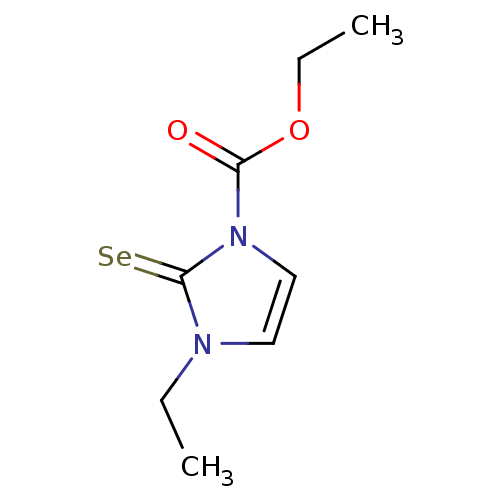
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
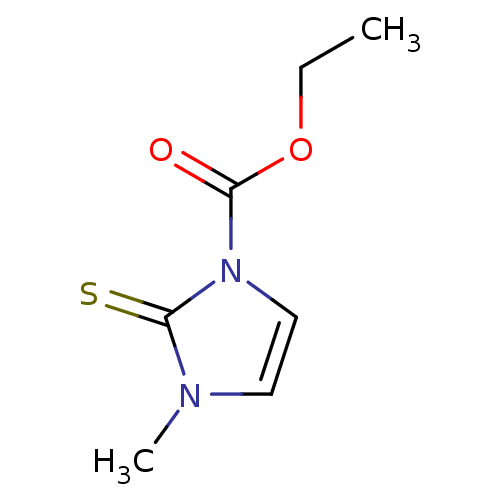
Affinity DataIC50: 1.01E+4nMAssay Description:Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLCMore data for this Ligand-Target Pair
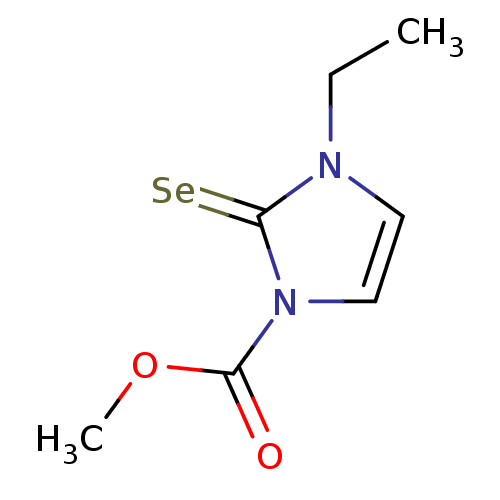
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
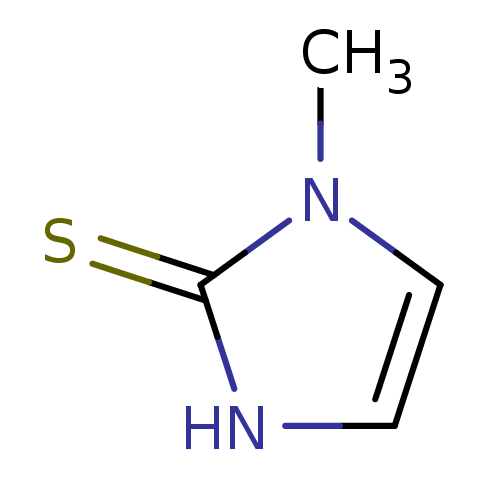
Affinity DataIC50: 1.04E+4nMAssay Description:Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLCMore data for this Ligand-Target Pair
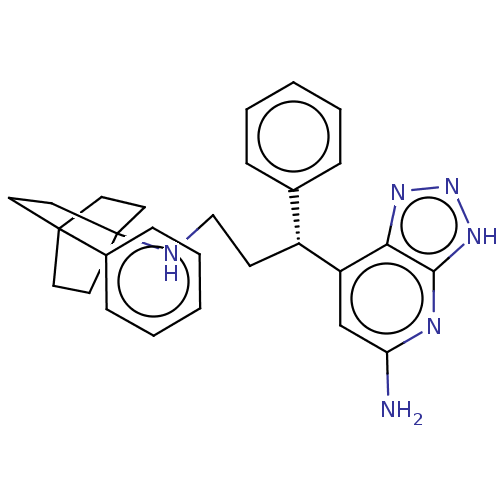
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
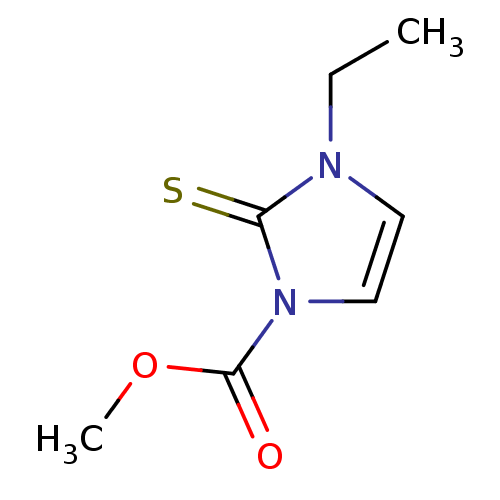
Affinity DataIC50: 1.07E+4nMAssay Description:Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLCMore data for this Ligand-Target Pair
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.18E+4nMAssay Description:Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLCMore data for this Ligand-Target Pair
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.96E+4nMAssay Description:Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLCMore data for this Ligand-Target Pair
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.97E+4nMAssay Description:Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLCMore data for this Ligand-Target Pair
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.05E+4nMAssay Description:Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLCMore data for this Ligand-Target Pair
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.13E+4nMAssay Description:Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLCMore data for this Ligand-Target Pair
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.53E+4nMAssay Description:Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLCMore data for this Ligand-Target Pair
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 6.20E+4nMAssay Description:Inhibition of LPO (unknown origin)More data for this Ligand-Target Pair
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 6.20E+4nMAssay Description:Inhibition of LPO (unknown origin)More data for this Ligand-Target Pair
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human LPO assessed as reduction in H2O2 catalyzed 3,5-iodo tyrosine formation from 3-iodotyrosine and potassium iodide preincubated for...More data for this Ligand-Target Pair