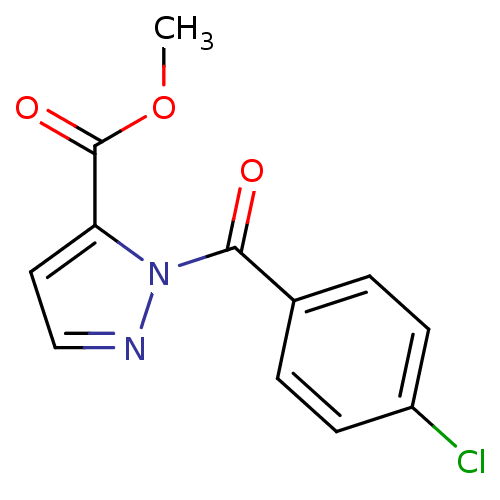
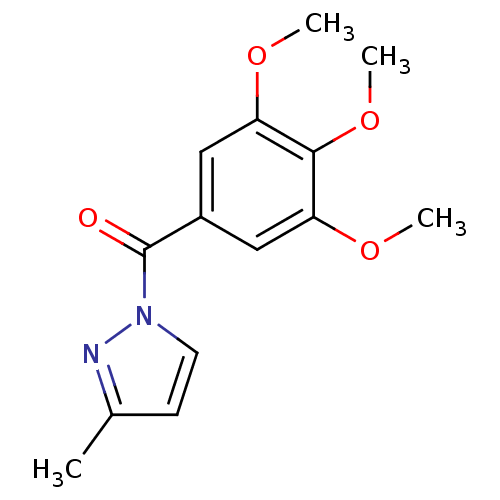
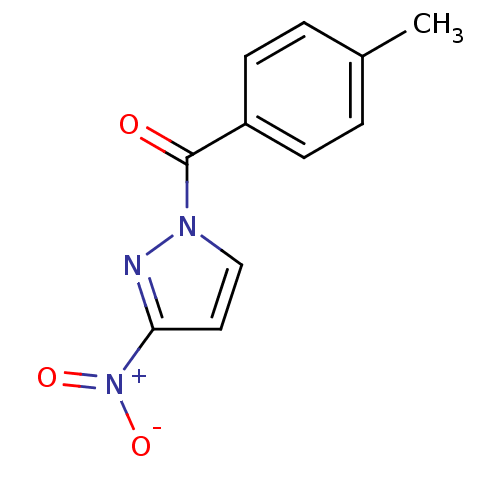
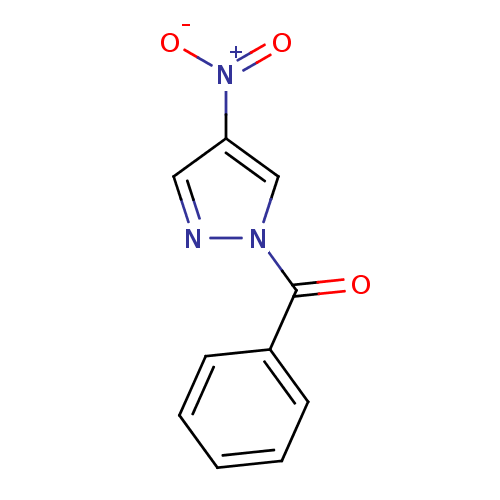
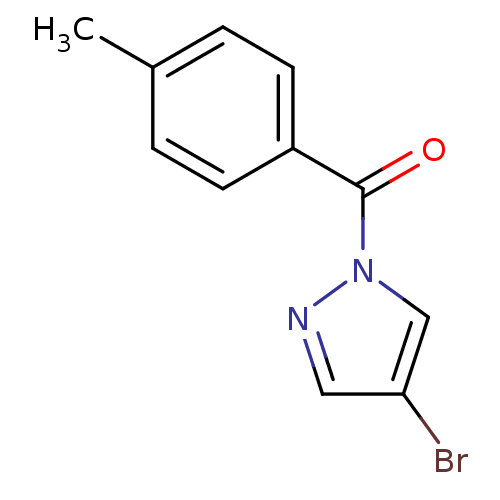
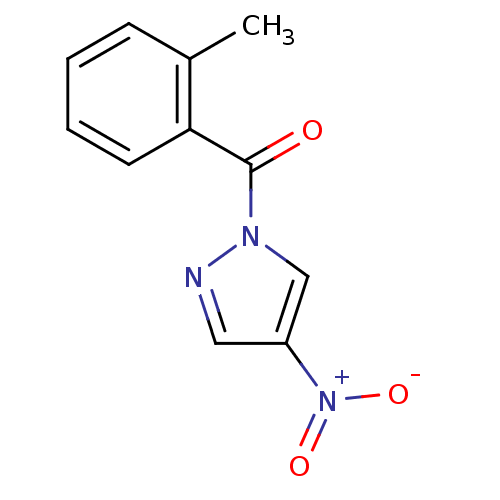
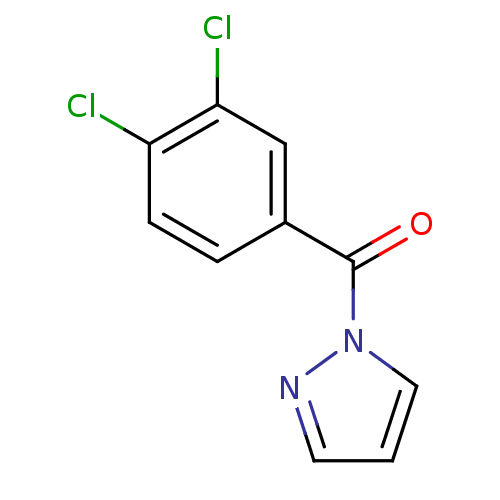
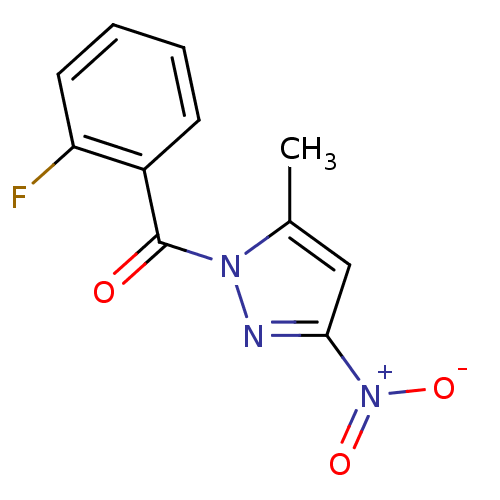
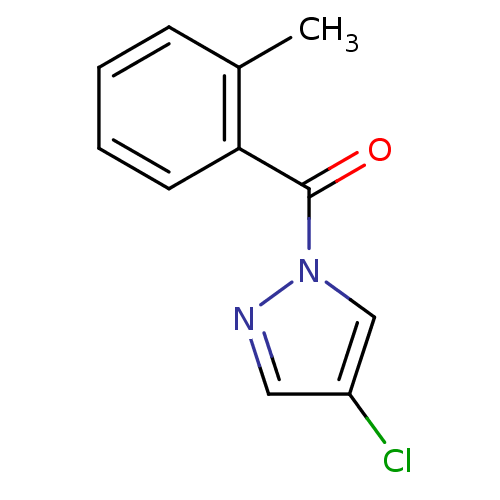
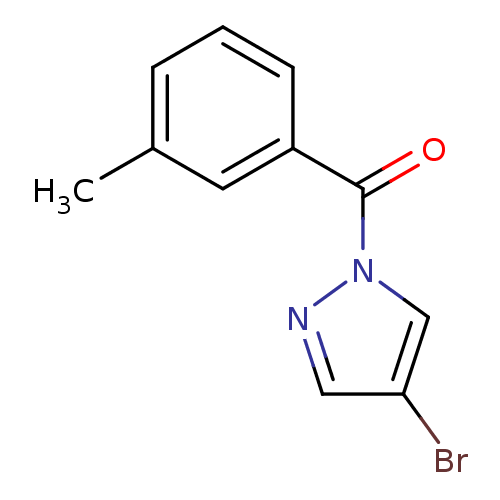
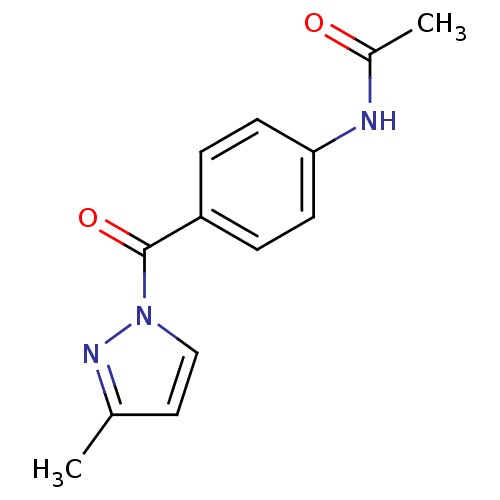
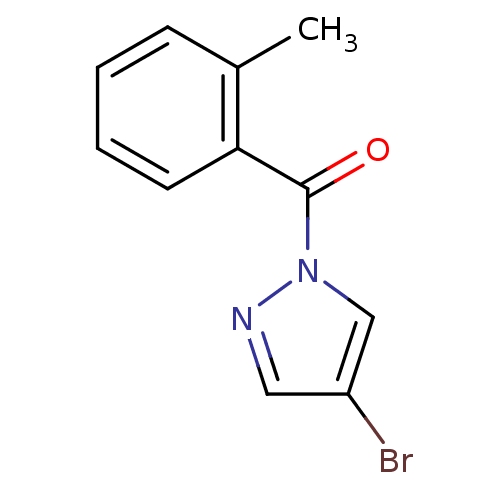
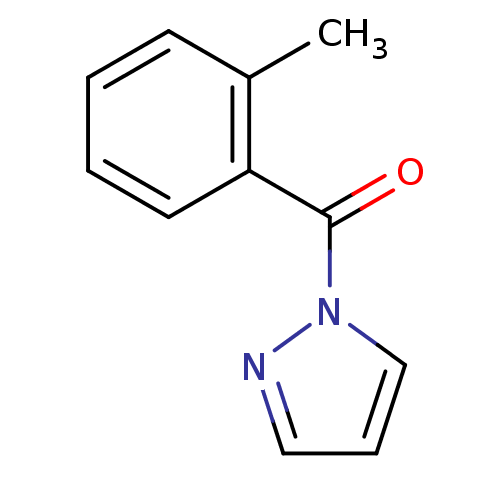
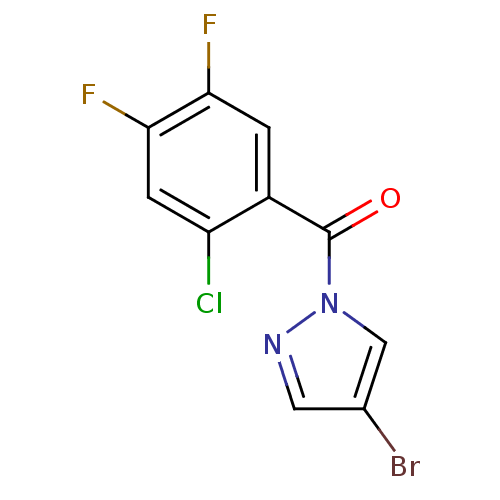
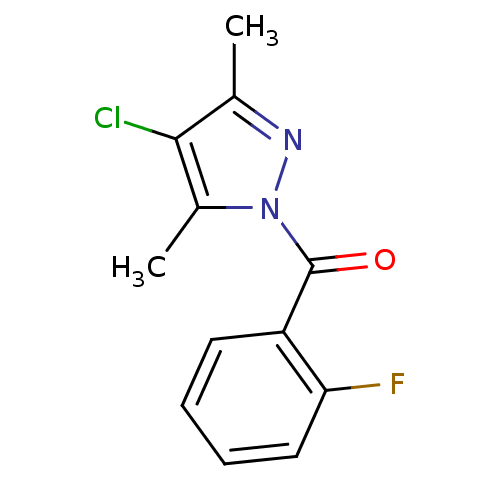
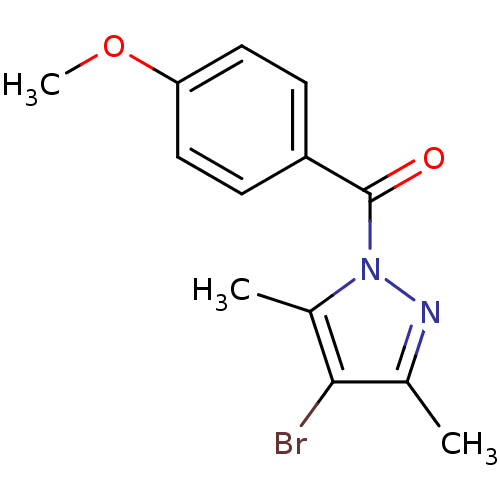
Affinity DataKi: 6nM ΔG°: -46.9kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 15nM ΔG°: -44.7kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 21nM ΔG°: -43.8kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 24nM ΔG°: -43.5kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 24nM ΔG°: -43.5kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 28nM ΔG°: -43.1kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 34nM ΔG°: -42.6kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 39nM ΔG°: -42.3kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 45nM ΔG°: -41.9kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 46nM ΔG°: -41.9kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 65nM ΔG°: -41.0kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 104nM ΔG°: -39.9kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 107nM ΔG°: -39.8kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 110nM ΔG°: -39.7kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 230nM ΔG°: -37.9kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 250nM ΔG°: -37.7kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 290nM ΔG°: -37.3kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 300nM ΔG°: -37.2kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 300nM ΔG°: -37.2kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 820nM ΔG°: -34.7kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nM ΔG°: -34.2kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nM ΔG°: -34.0kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 3.10E+3nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 3.40E+3nM ΔG°: -31.2kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 7.20E+3nM ΔG°: -29.4kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 9.00E+3nM ΔG°: -28.8kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 9.00E+3nM ΔG°: -28.8kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 1.07E+4nM ΔG°: -28.4kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 2.45E+4nM ΔG°: -26.3kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 2.99E+4nM ΔG°: -25.8kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 5.09E+4nM ΔG°: -24.5kJ/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o...More data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Urokinase activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration ...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o...More data for this Ligand-Target Pair
Affinity DataIC50: 125nMAssay Description:Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati...More data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:Urokinase activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration ...More data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati...More data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati...More data for this Ligand-Target Pair
Affinity DataIC50: 340nMAssay Description:Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati...More data for this Ligand-Target Pair
Affinity DataIC50: 390nMAssay Description:Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o...More data for this Ligand-Target Pair
Affinity DataIC50: 680nMAssay Description:Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Urokinase activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration ...More data for this Ligand-Target Pair