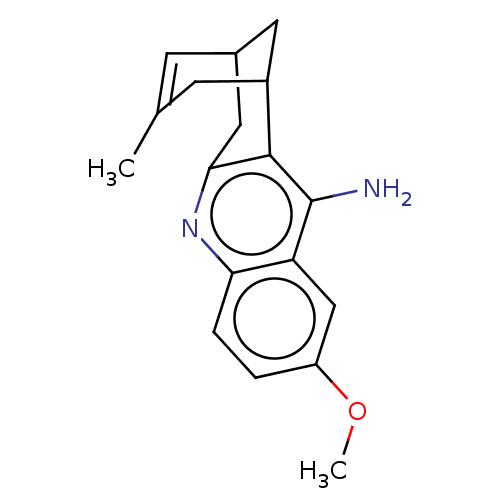
Affinity DataKi: 23nMAssay Description:Non competitive type inhibition of human AChE assessed as inhibition constant using varying levels of acetylthiocholine as substrate by double recipr...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9.10nMAssay Description:Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 85nMAssay Description:Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 320nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 358nMAssay Description:Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.78E+3nMAssay Description:Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.63E+3nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.76E+3nMAssay Description:Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.76E+4nMAssay Description:Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+4nMAssay Description:Inhibition of recombinant human nNOSMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibition of recombinant human nNOSMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+4nMAssay Description:Inhibition of recombinant human nNOSMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of recombinant human nNOSMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+4nMAssay Description:Inhibition of recombinant human nNOSMore data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+4nMAssay Description:Inhibition of recombinant human nNOSMore data for this Ligand-Target Pair

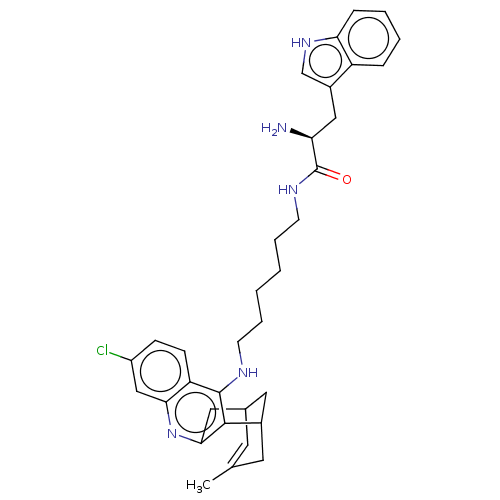


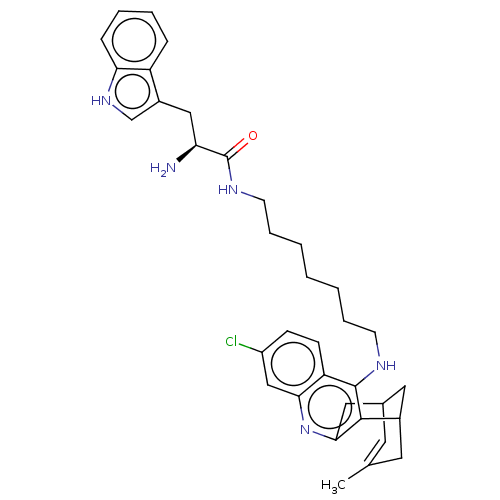

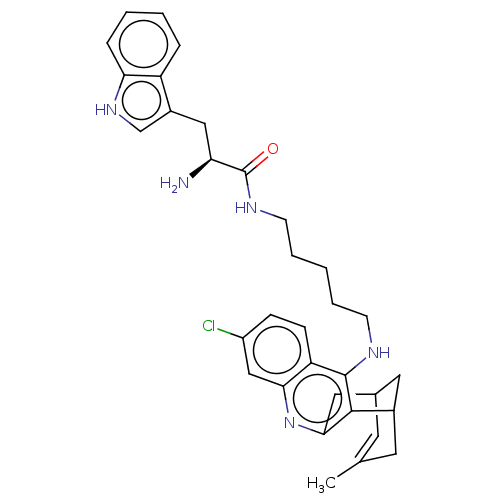

 3D Structure (crystal)
3D Structure (crystal)