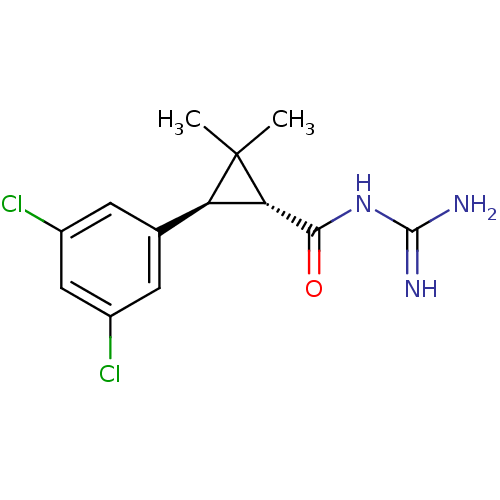
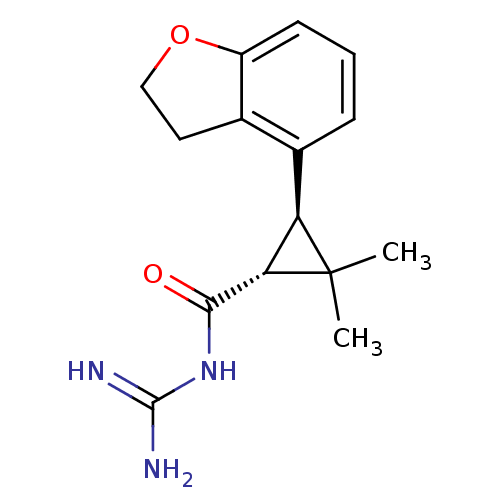
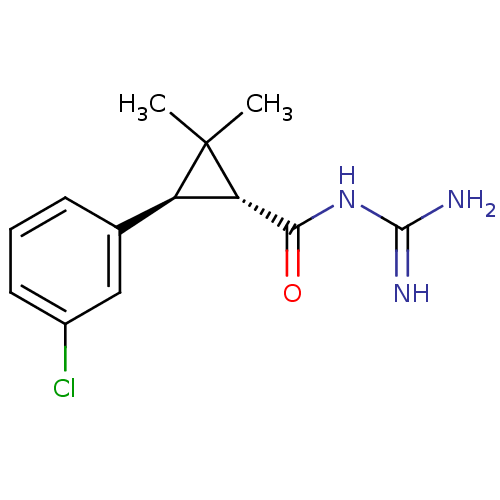
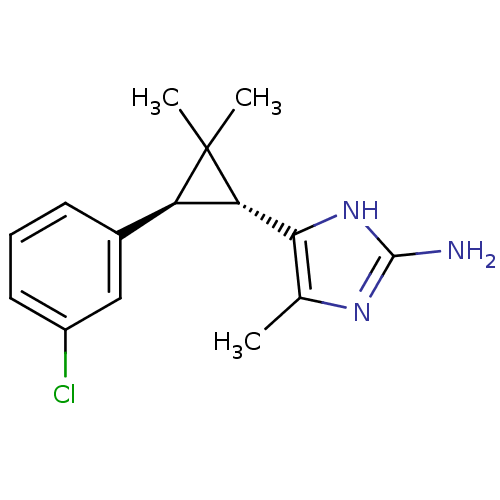
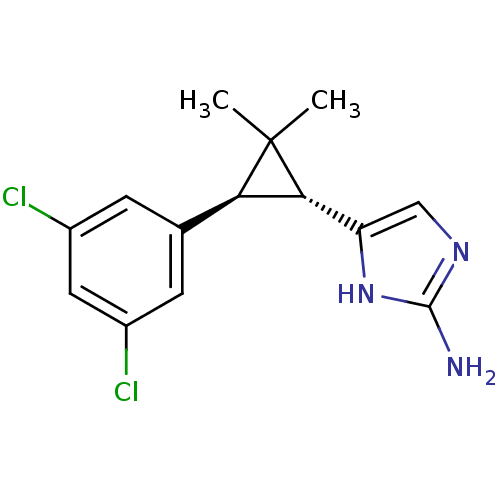
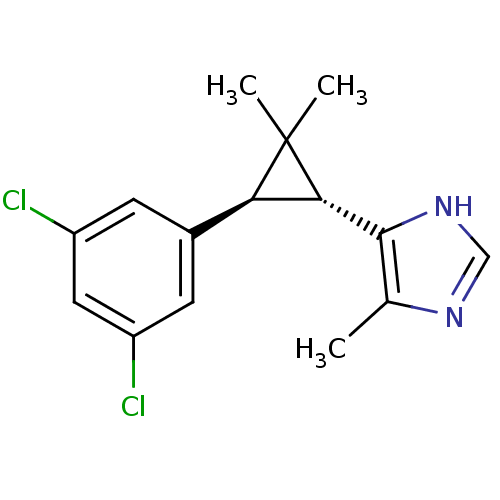
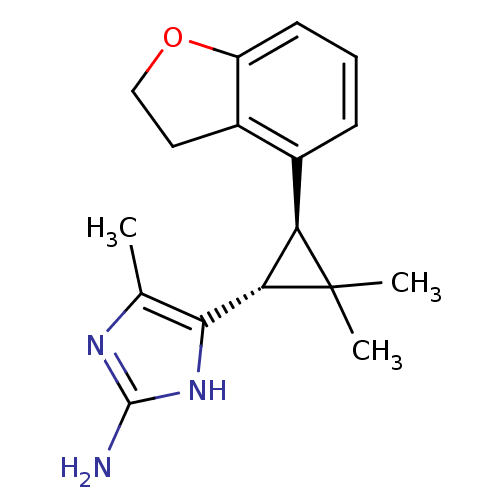
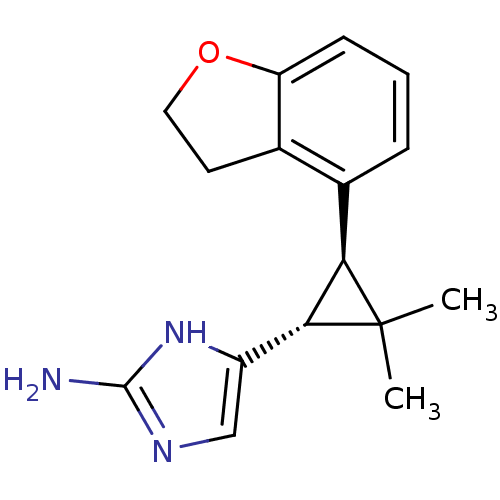
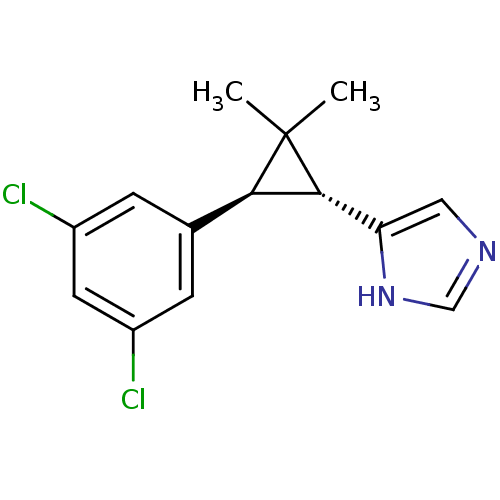
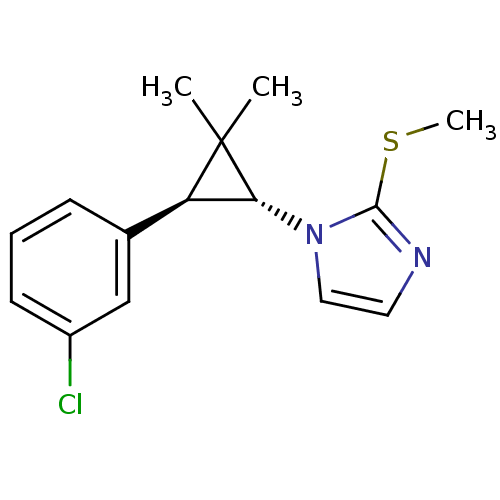
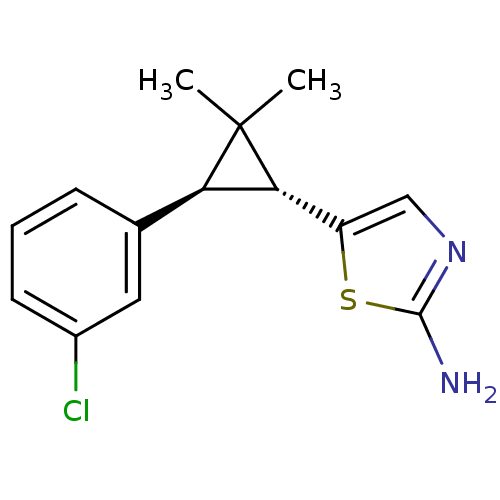
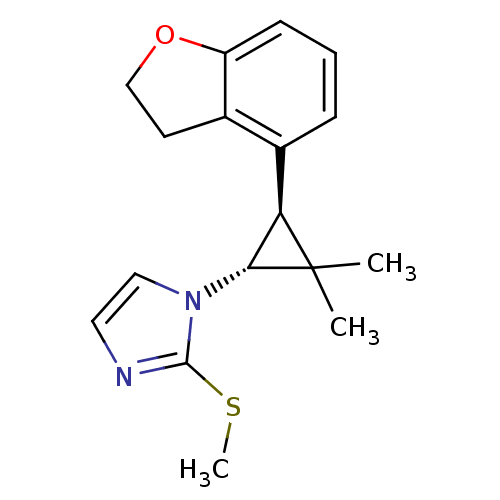
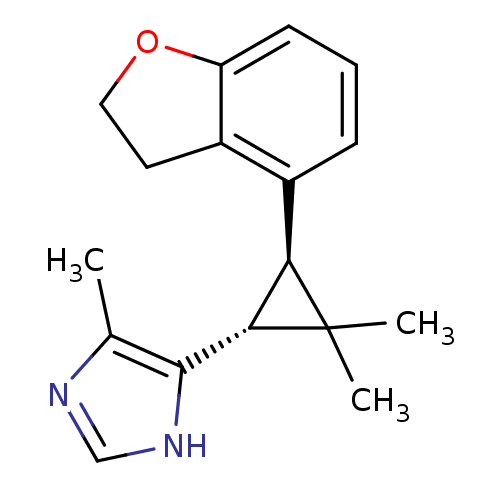
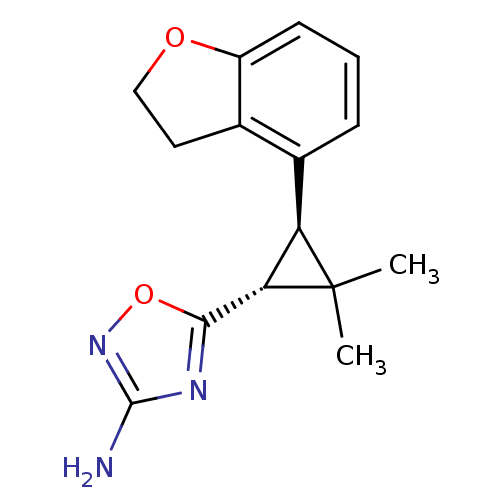
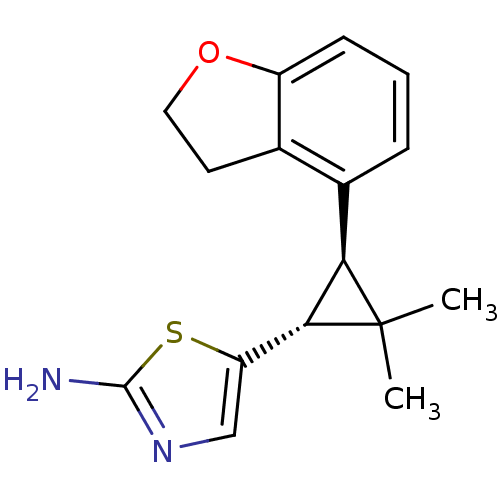
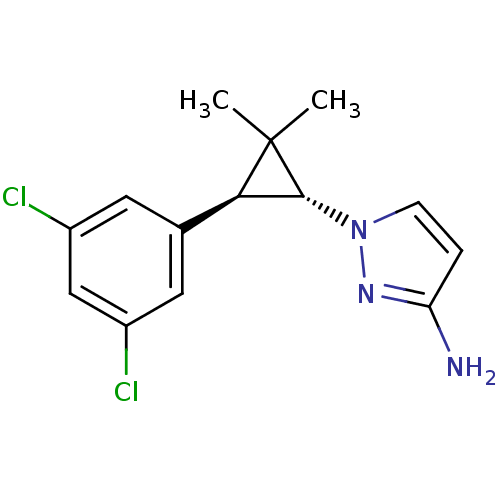
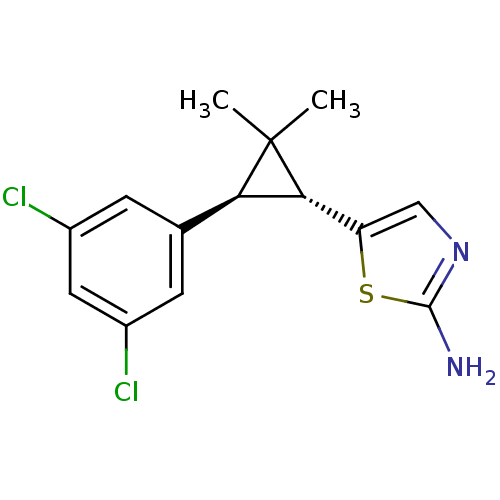
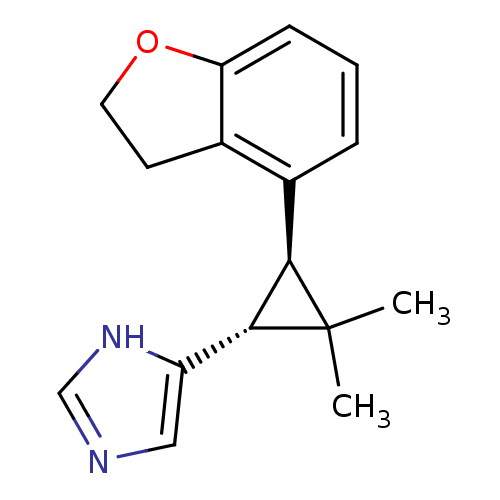
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 39nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 750nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 950nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.85E+3nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of sodium dependent recovery of pH following imposed acidosis in AP1 cell line expressing the human NHE-1 isoform.More data for this Ligand-Target Pair