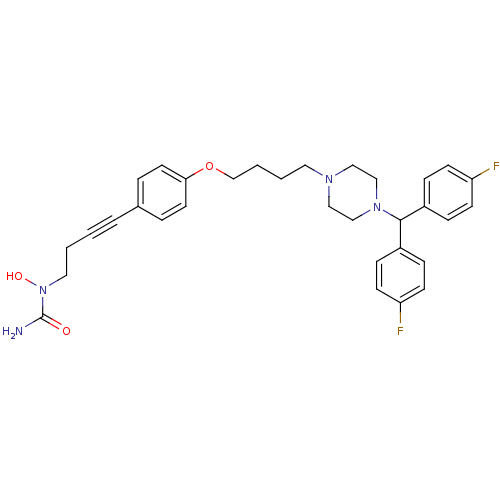
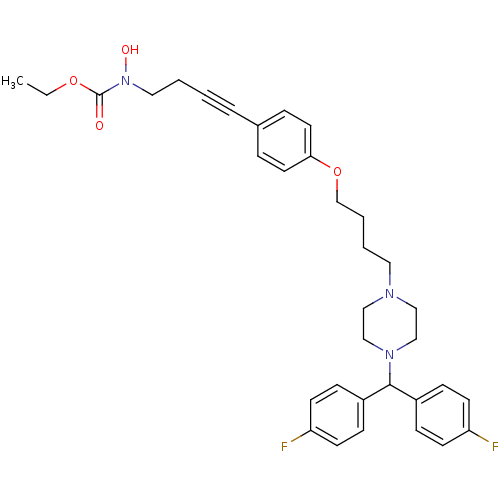
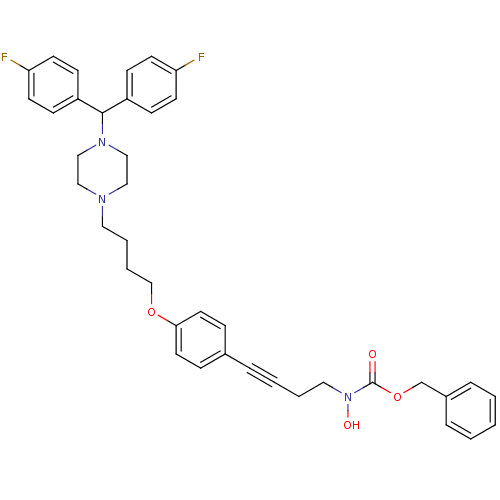
Affinity DataKi: 3.63nMAssay Description:Binding affinity for recombinant human Histamine H1 receptor expressed in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.01nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.89nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.89nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 7.59nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 9.55nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 17.8nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:Inhibitory concentration against 5-lipoxygenase in human whole bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 89nMAssay Description:Inhibitory concentration against 5-lipoxygenase in human whole bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibitory concentration against 5-lipoxygenase in human whole bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 102nMAssay Description:Inhibitory concentration against 5-lipoxygenase in human whole bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibitory concentration against 5-lipoxygenase in human whole bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:Inhibitory concentration against human 5-lipoxygenaseMore data for this Ligand-Target Pair
Affinity DataIC50: 176nMAssay Description:Inhibitory concentration against 5-lipoxygenase in human whole bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 193nMAssay Description:Inhibitory concentration against human 5-lipoxygenaseMore data for this Ligand-Target Pair
Affinity DataIC50: 335nMAssay Description:Inhibitory concentration against human 5-lipoxygenaseMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibitory concentration against human 5-lipoxygenaseMore data for this Ligand-Target Pair
Affinity DataIC50: 365nMAssay Description:Inhibitory concentration against human 5-lipoxygenaseMore data for this Ligand-Target Pair
Affinity DataIC50: 420nMAssay Description:Inhibitory concentration against human 5-lipoxygenaseMore data for this Ligand-Target Pair
Affinity DataIC50: 518nMAssay Description:Inhibitory concentration against human 5-lipoxygenaseMore data for this Ligand-Target Pair
Affinity DataIC50: 873nMAssay Description:Inhibitory concentration against 5-lipoxygenase in human whole bloodMore data for this Ligand-Target Pair