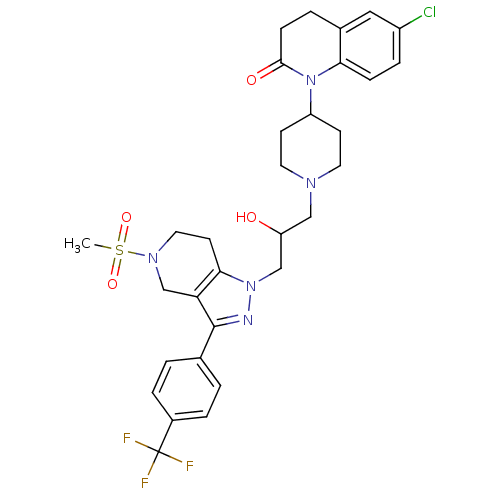
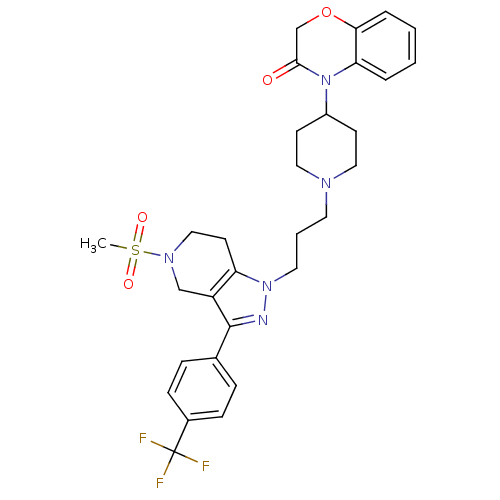
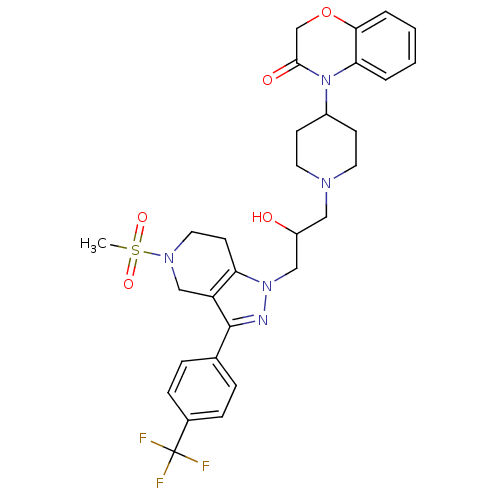
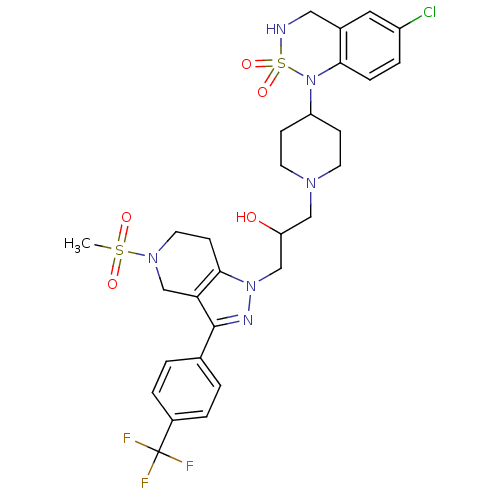
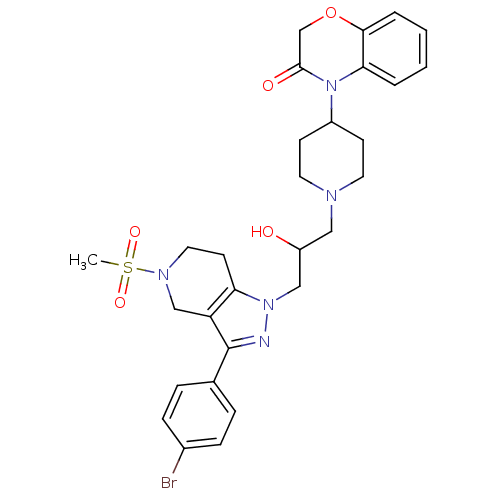
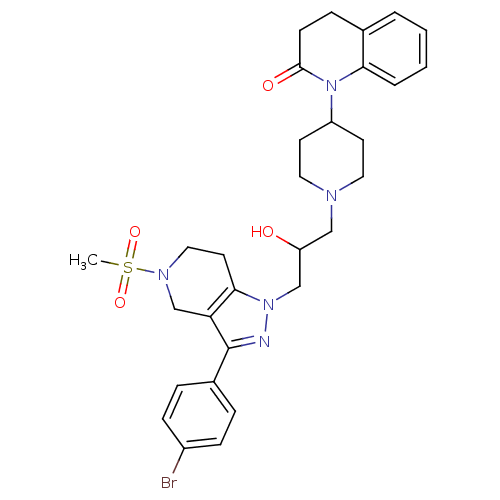
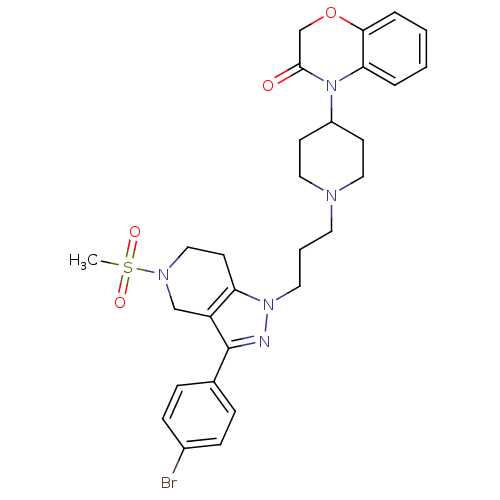
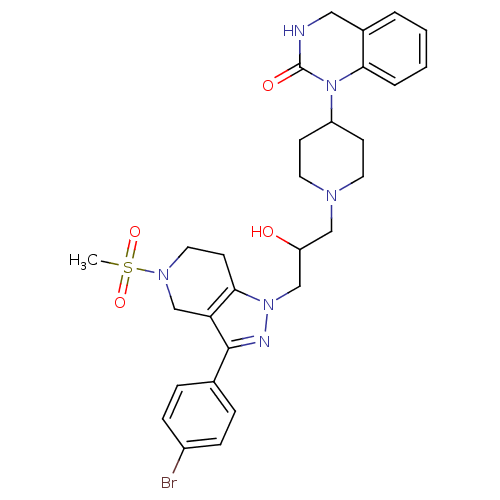
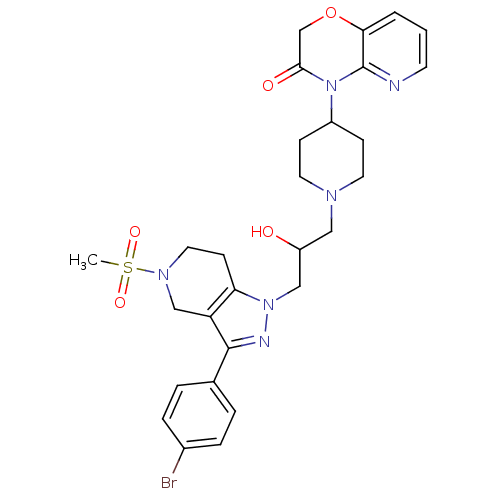
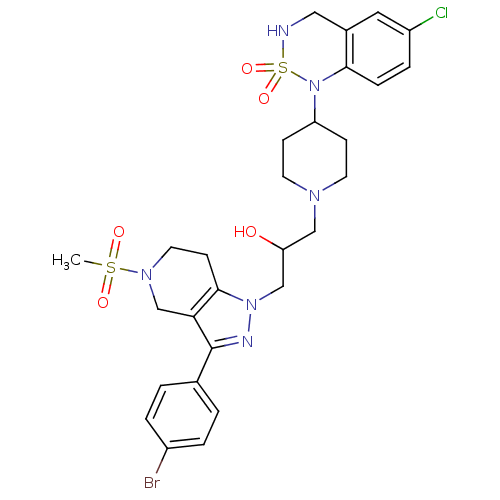
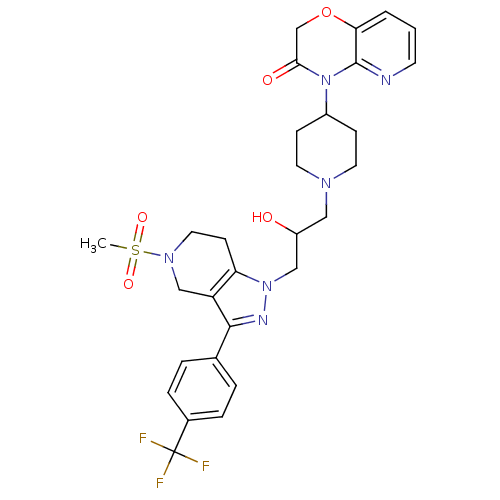
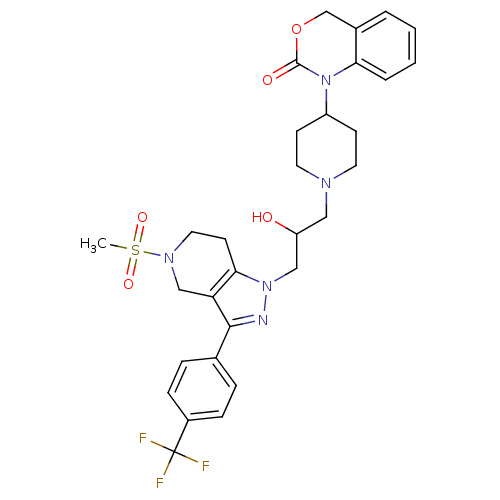
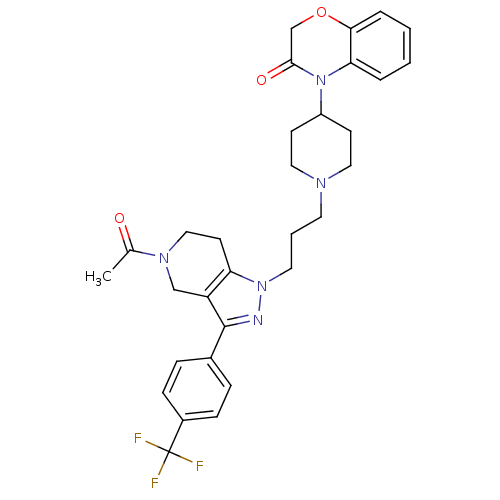
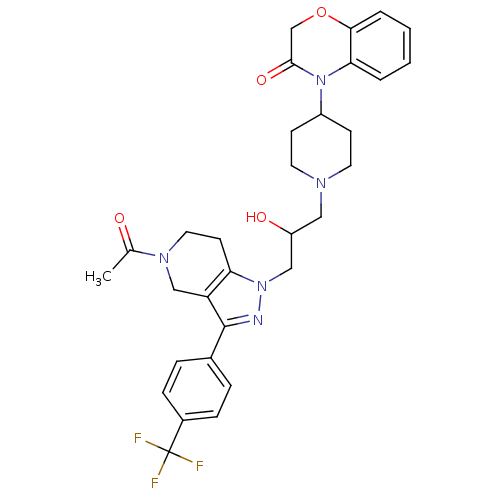
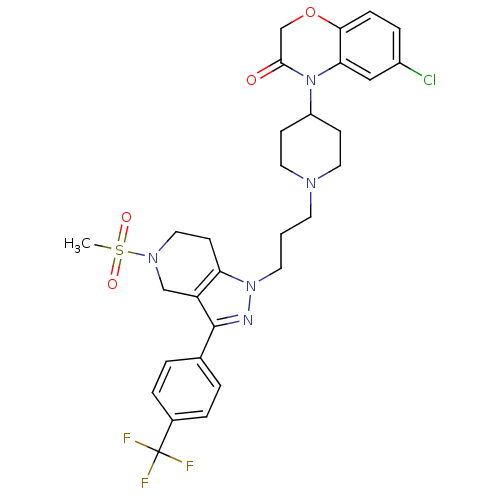
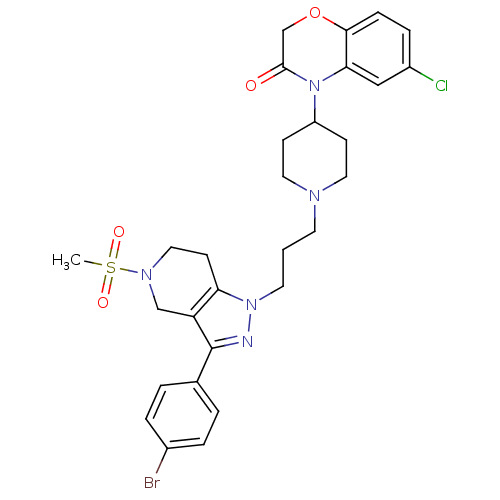
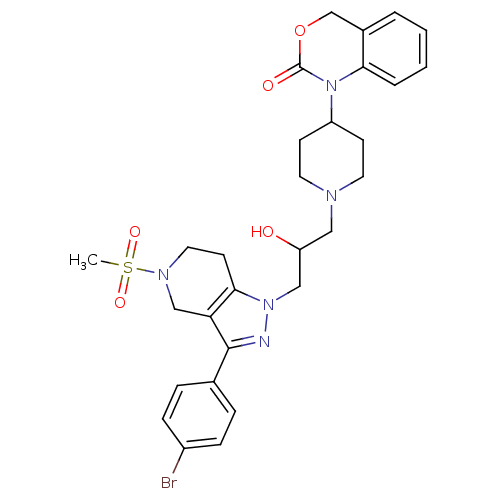
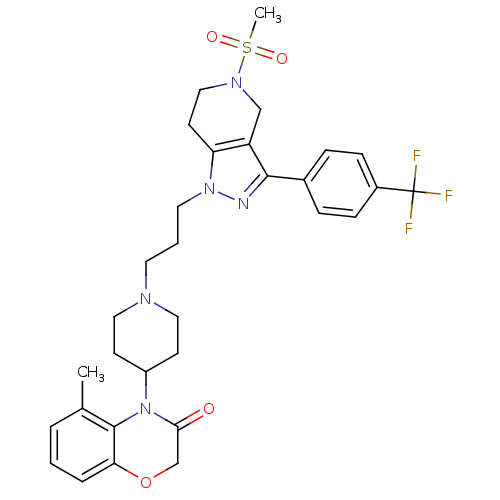
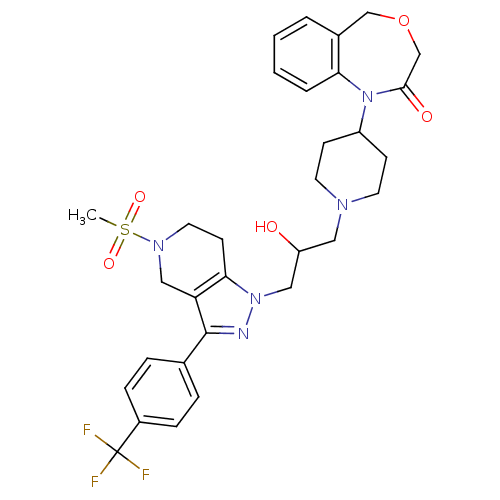
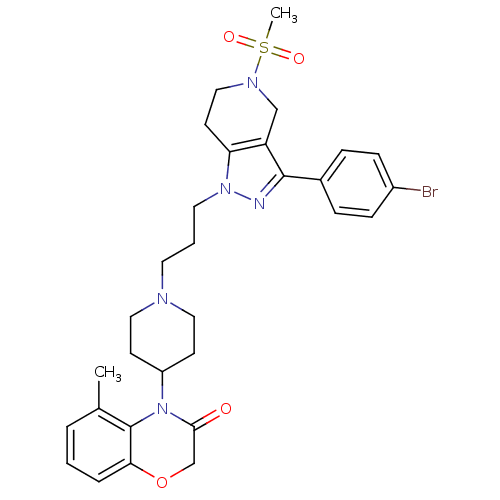
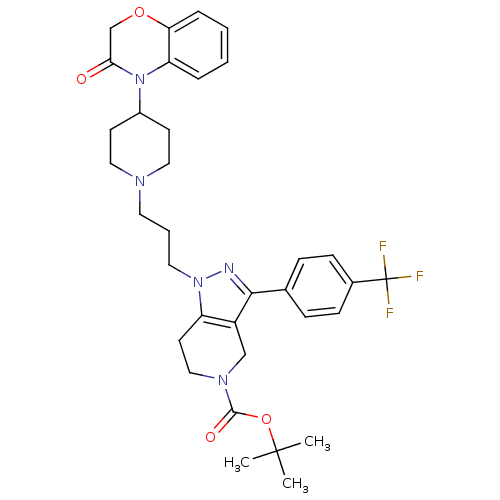
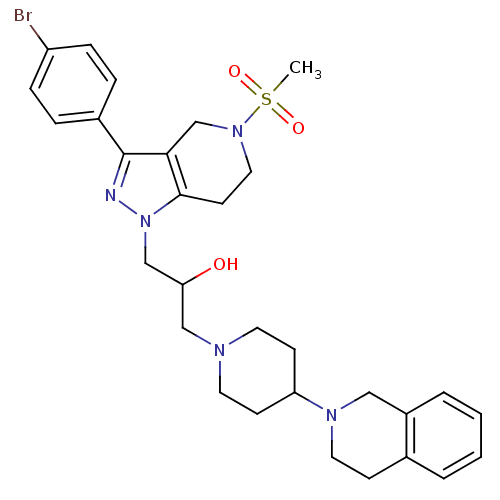
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
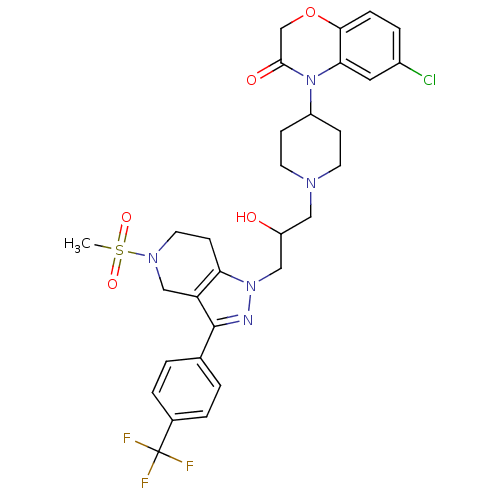
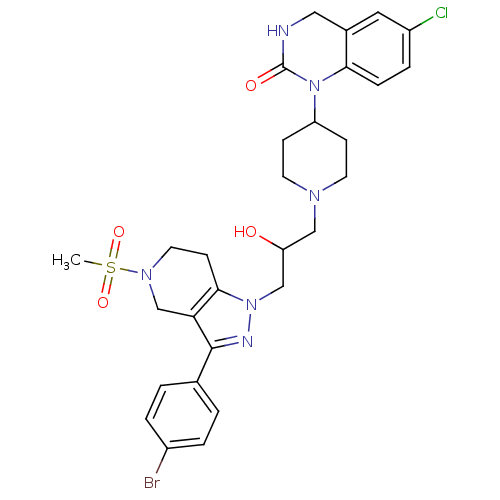
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
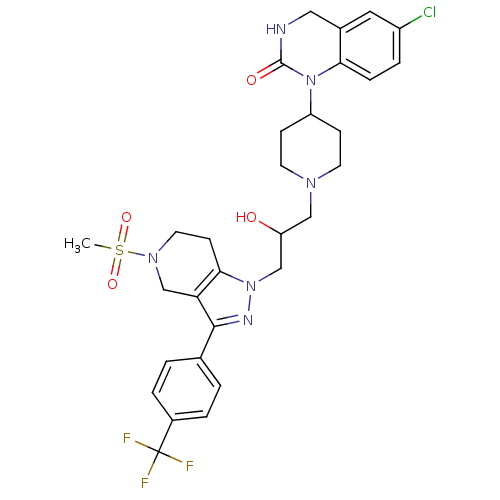
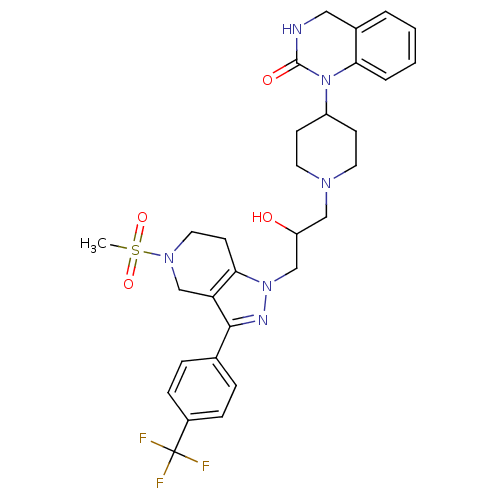
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
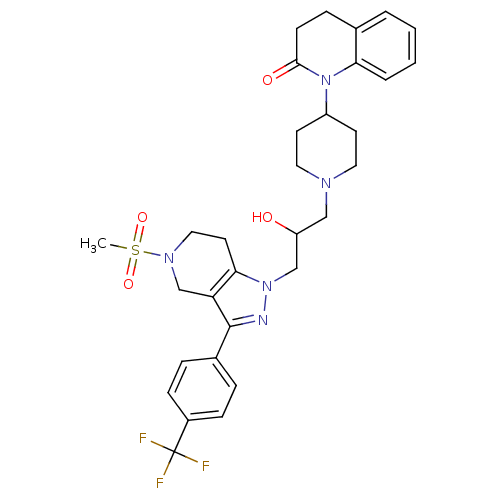
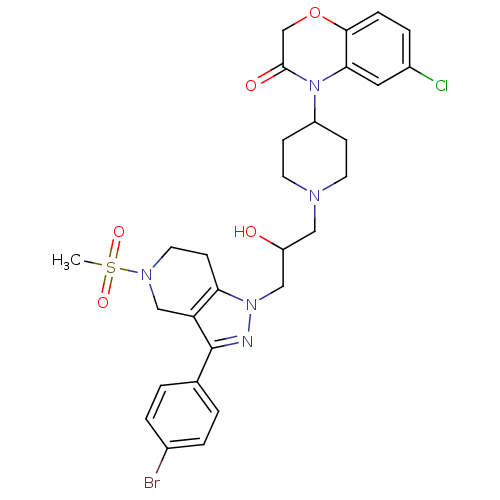
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 68nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 75nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 88nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 133nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 145nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 163nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 322nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 410nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 750nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 810nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 826nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 930nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 950nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 980nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetHLA class II histocompatibility antigen gamma chain(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of MHC2 invariant chainMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.78E+3nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair
TargetCathepsin S(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of cathepsin SMore data for this Ligand-Target Pair