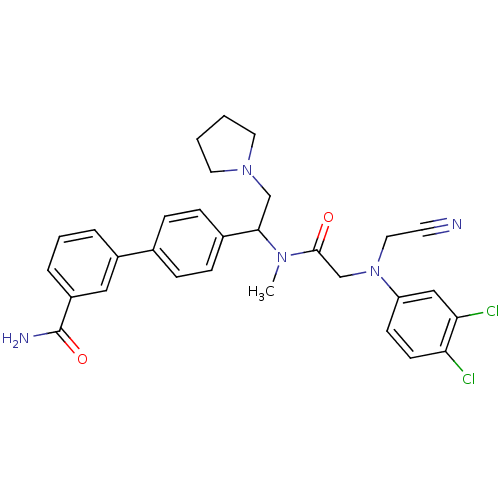
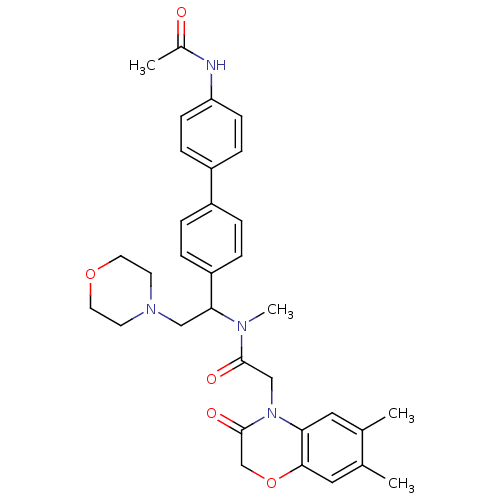
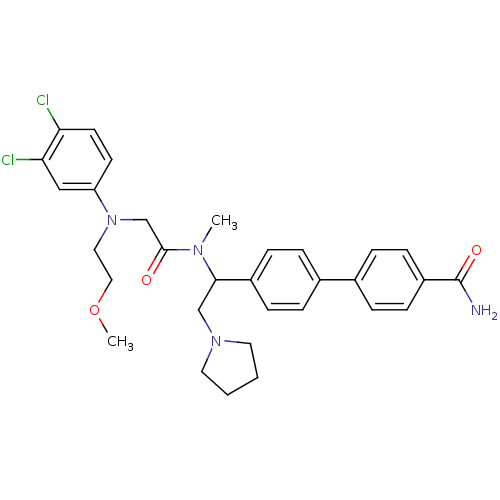
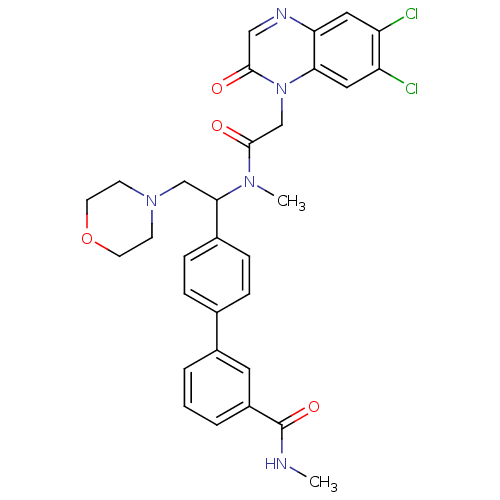
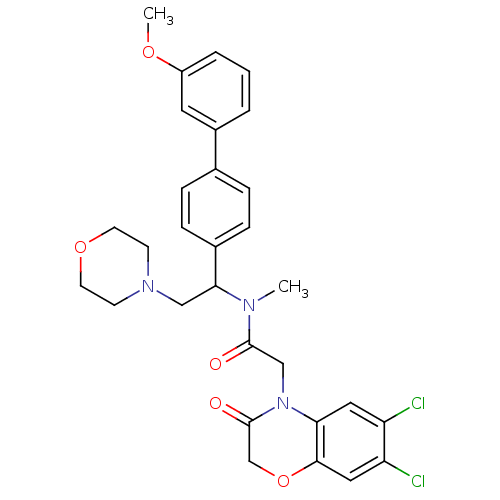
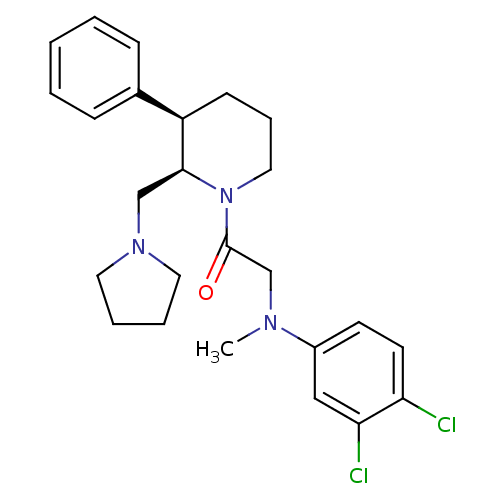
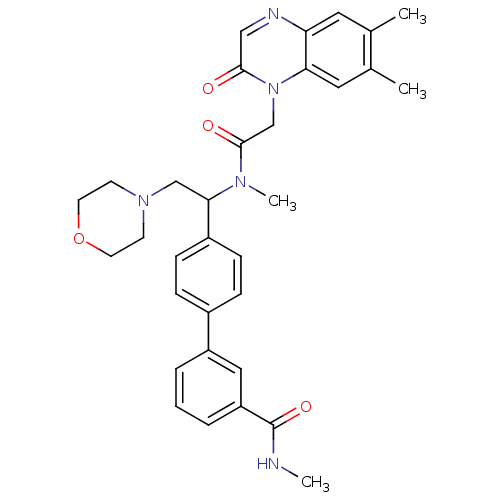
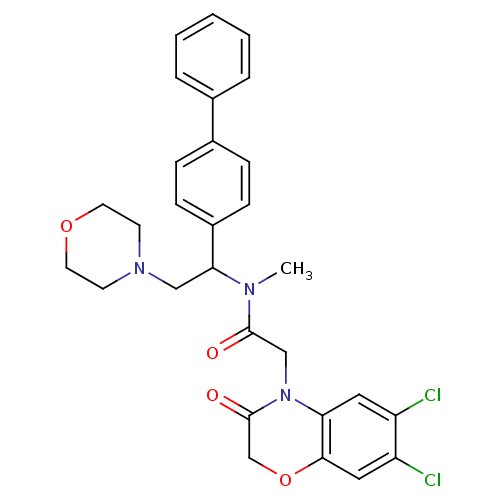
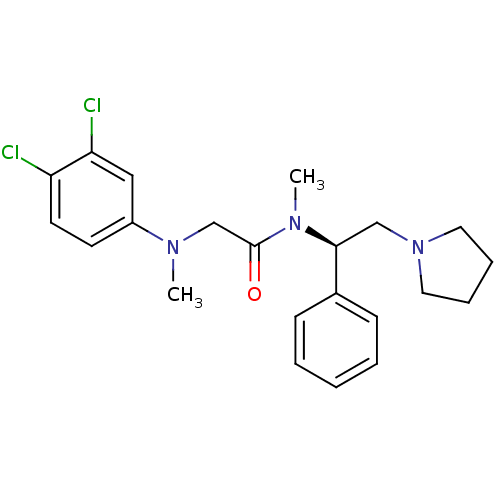
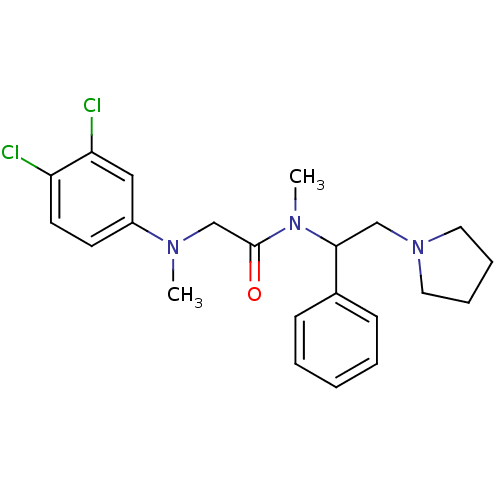
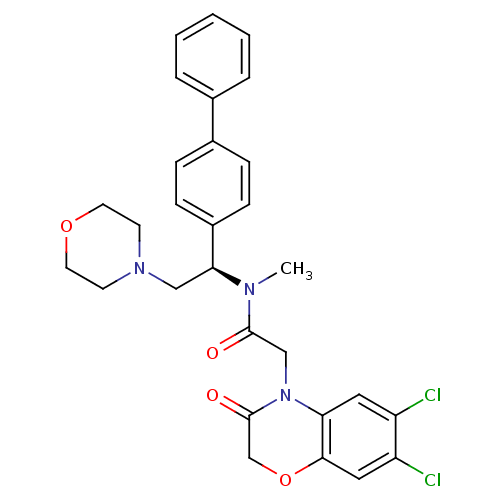
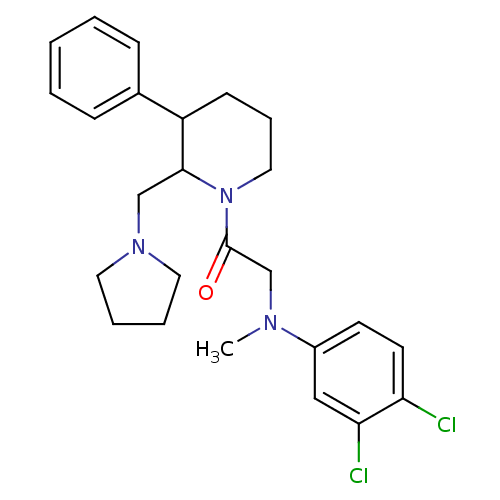
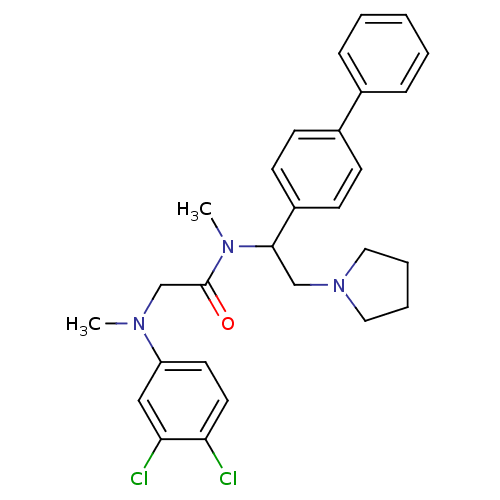
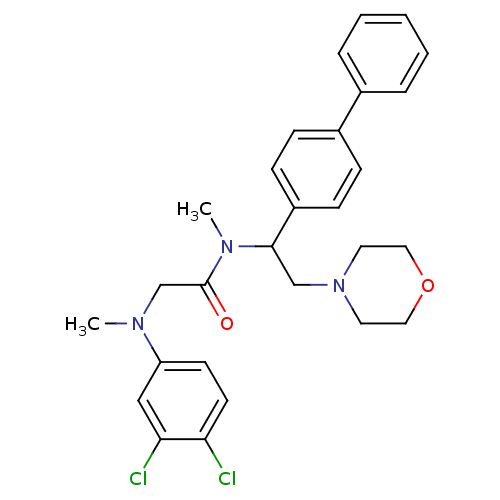
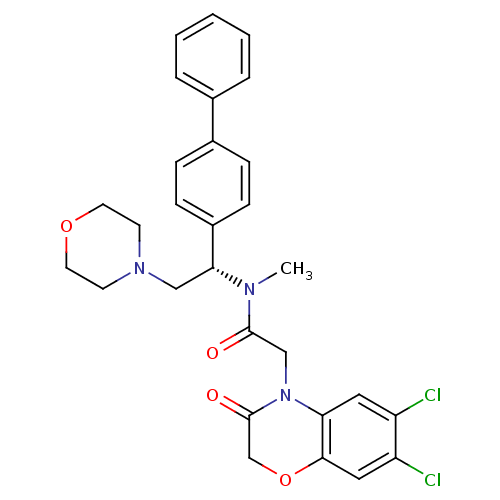
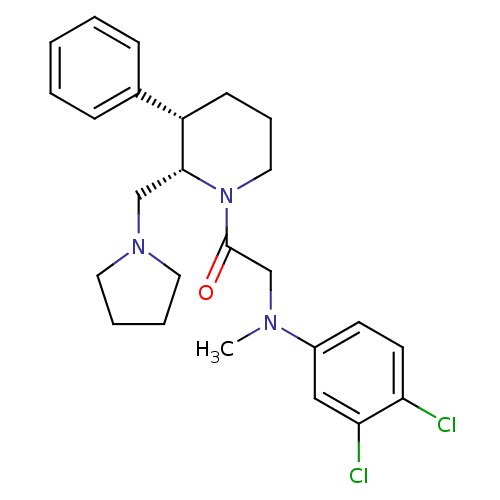
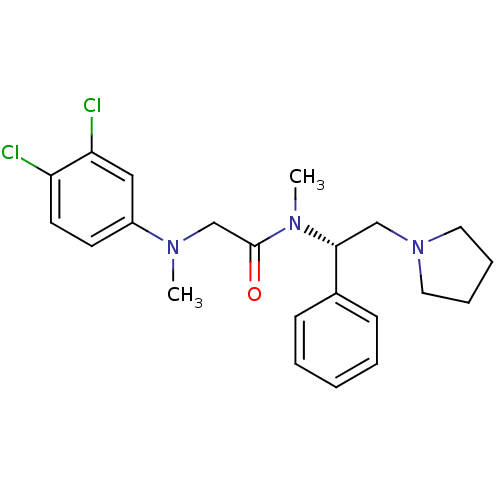
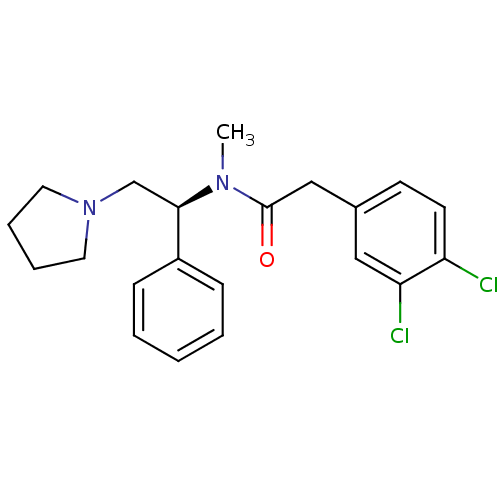
Affinity DataKi: 0.300nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.60E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+3nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 9.10E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 4.00E+3nMAssay Description:Agonist activity at kappa opioid receptor (unknown origin)More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 100nMAssay Description:Agonist activity at kappa opioid receptor (unknown origin)More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 32nMAssay Description:Agonist activity at kappa opioid receptor (unknown origin)More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 2.00E+3nMAssay Description:Agonist activity at kappa opioid receptor (unknown origin)More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 800nMAssay Description:Agonist activity at kappa opioid receptor (unknown origin)More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 160nMAssay Description:Agonist activity at kappa opioid receptor (unknown origin)More data for this Ligand-Target Pair