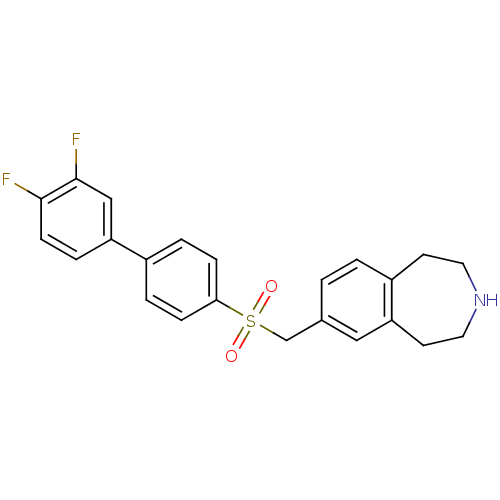
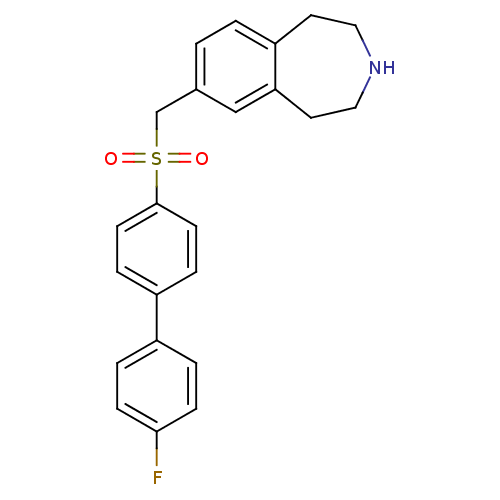
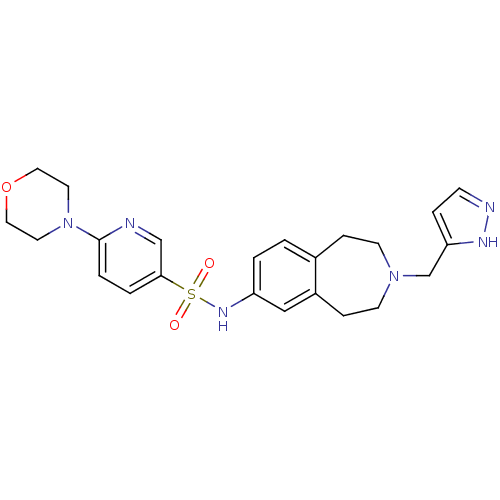
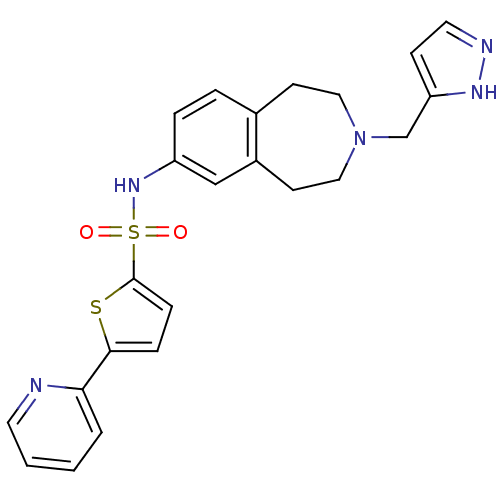
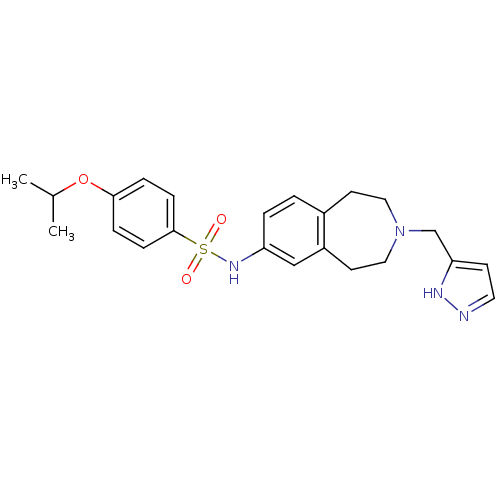
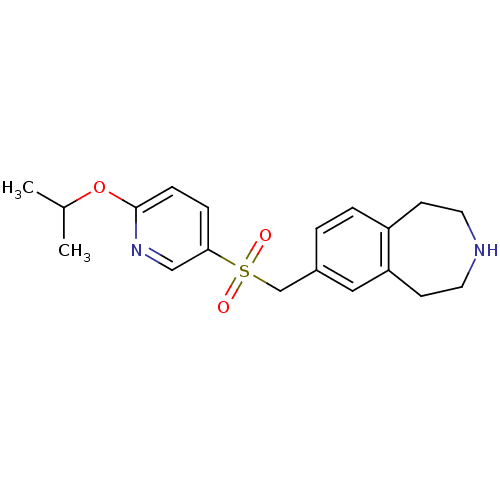
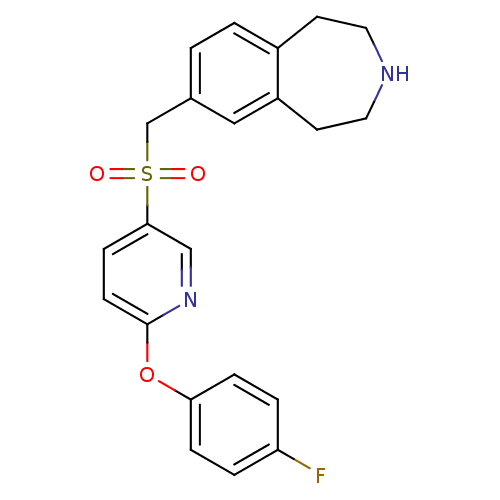
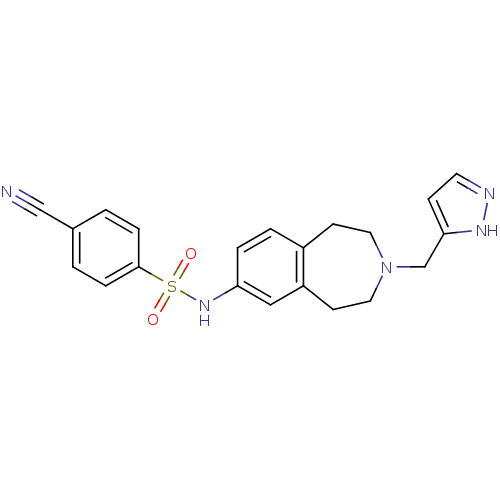
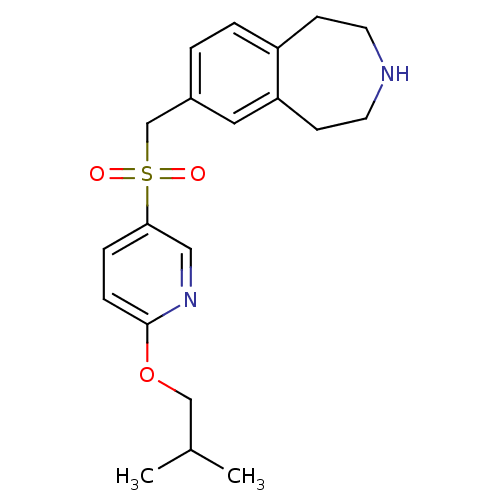
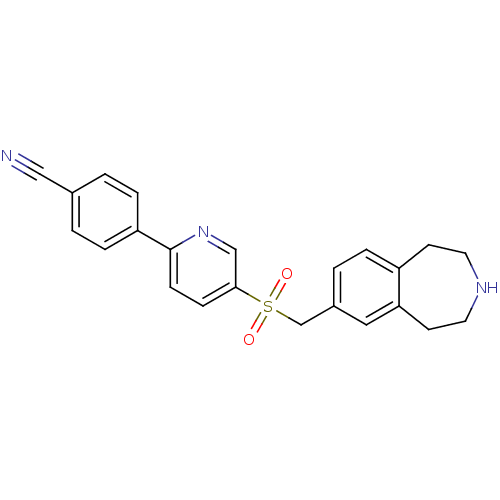
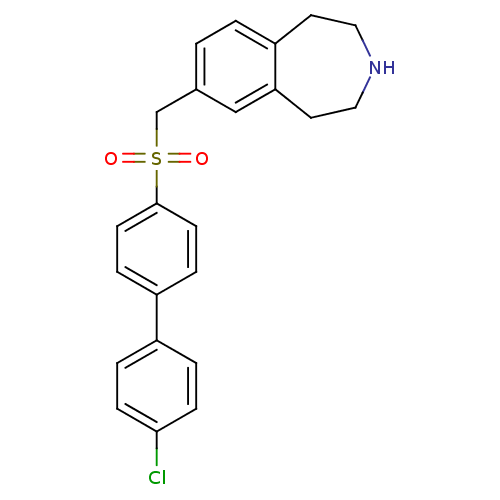
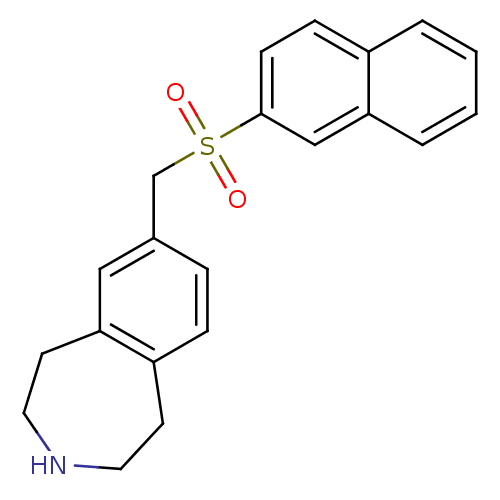
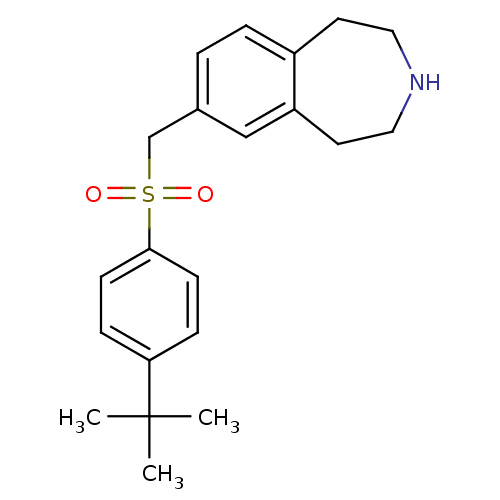
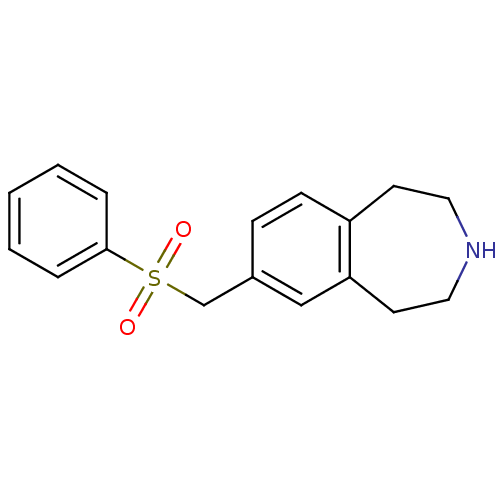
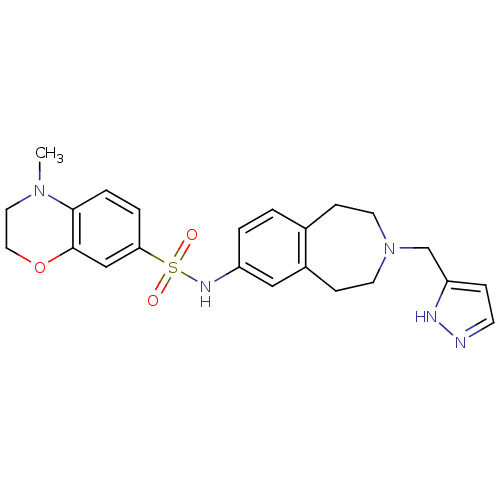
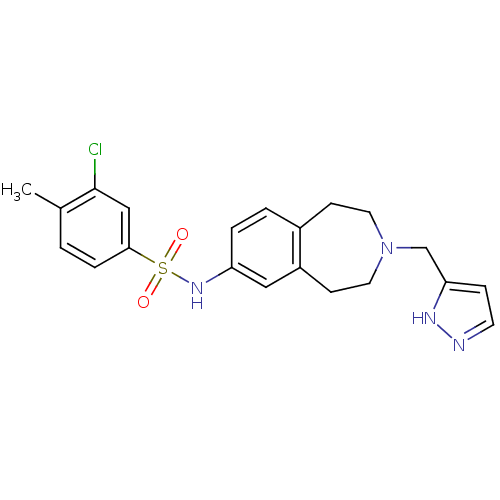
Affinity DataIC50: 100nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human recombinant CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of human recombinant CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of human recombinant CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of human recombinant CYP3A4 using diethoxyfluorescein as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-{3-(4-phenylpiperazin-1-ylmethyl)benzyl}resorufin as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human recombinant CYP2C9More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 2.51E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90E+3nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of human recombinant CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-{3-(4-phenylpiperazin-1-ylmethyl)benzyl}resorufin as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using diethoxyfluorescein as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 8.80E+3nMAssay Description:Inhibition of human recombinant CYP2C19More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 1.58E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+4nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: 631nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: 158nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: 1.58E+3nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: 79.4nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human gherlin receptorMore data for this Ligand-Target Pair