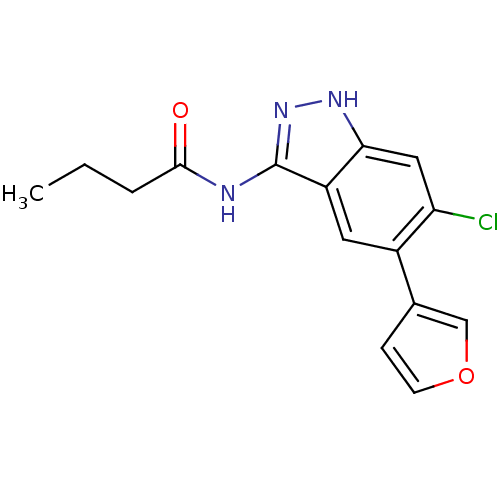
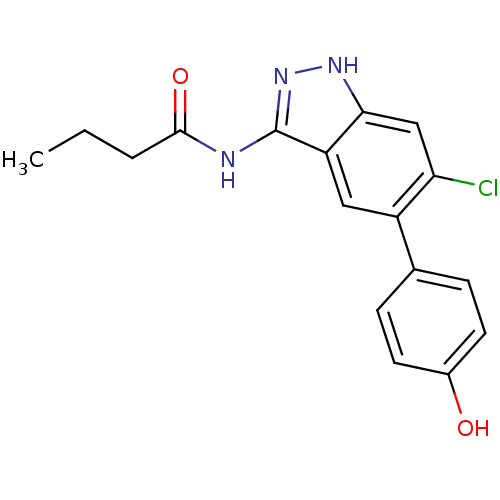
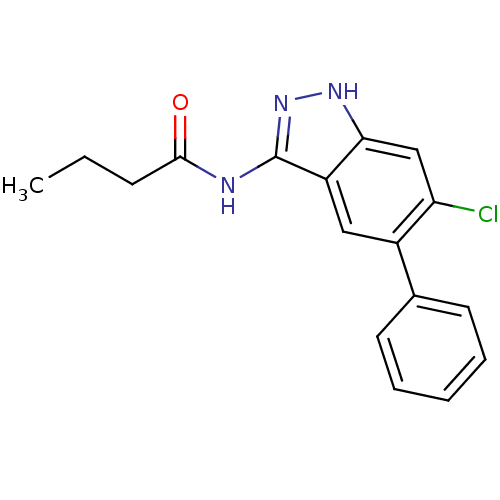
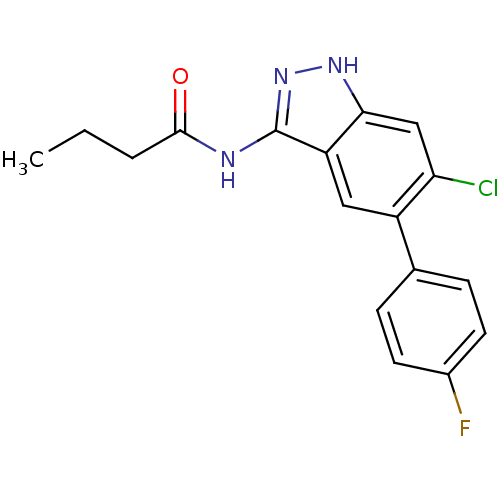
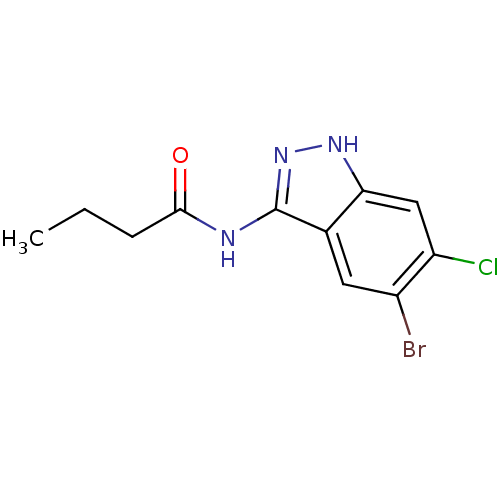
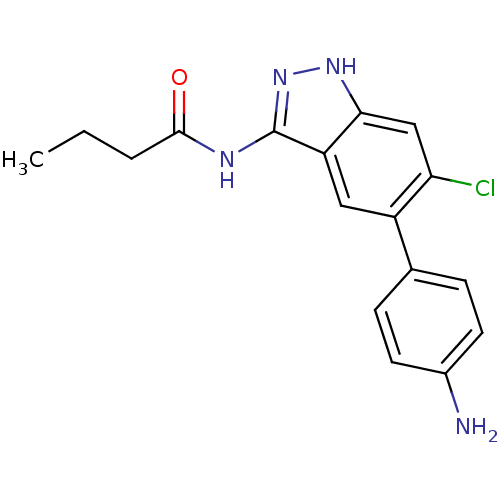
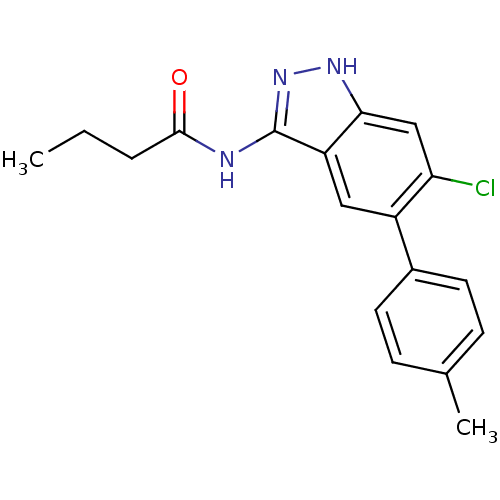
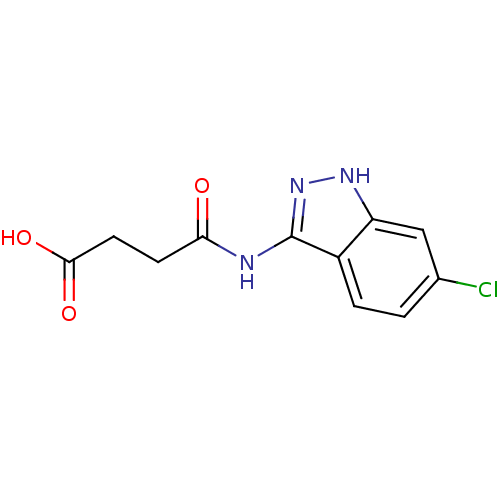
Affinity DataIC50: 30nMAssay Description:Inhibition of human recombinant GSK3beta by kinetic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of human recombinant GSK3beta by kinetic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of human recombinant GSK3beta by kinetic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of human recombinant GSK3beta by kinetic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of human recombinant GSK3beta by kinetic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:Inhibition of GSK3beta assessed as tau phosphorylationMore data for this Ligand-Target Pair
Affinity DataIC50: 390nMAssay Description:Inhibition of human recombinant GSK3beta by kinetic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 830nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 980nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.75E+3nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human recombinant GSK3beta by kinetic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.46E+3nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibition of human recombinant GSK3beta by kinetic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Sanofi-aventis
Curated by ChEMBL
Sanofi-aventis
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.38E+4nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of human recombinant GSK3beta by kinetic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair