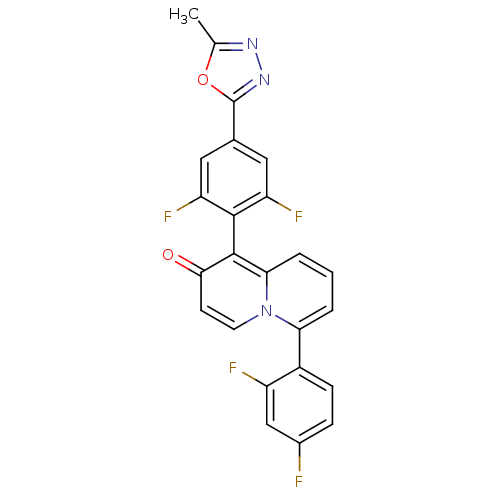
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 870nMAssay Description:Displacement of labeled MK-499 from human ERG in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 5.10E+3nMAssay Description:Displacement of labeled MK-499 from human ERG in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+4nMAssay Description:Displacement of labeled MK-499 from human ERG in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+4nMAssay Description:Displacement of labeled MK-499 from human ERG in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+4nMAssay Description:Displacement of labeled MK-499 from human ERG in HEK293 cellsMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.30nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.80nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 7.10nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 8.80nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 14.7nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of mouse p38alpha after 3 hrs by SPA methodMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of PXRMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of PXRMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of PXRMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of PXRMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of PXRMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of PXRMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of PXRMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: 3.30E+3nMAssay Description:Activation of PXRMore data for this Ligand-Target Pair

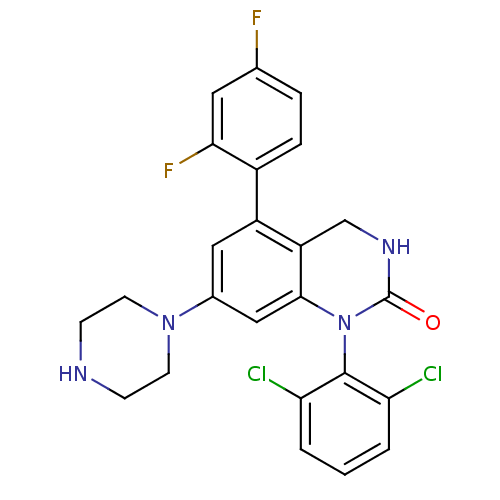

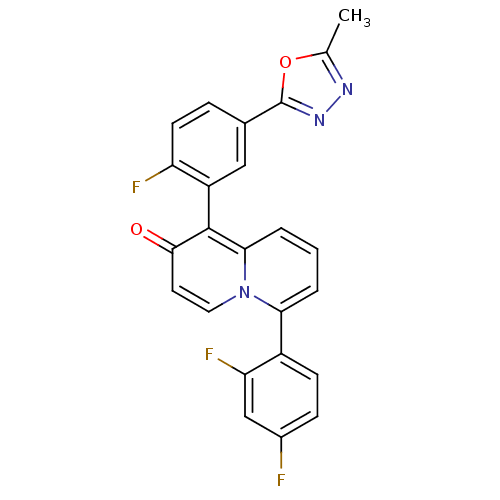
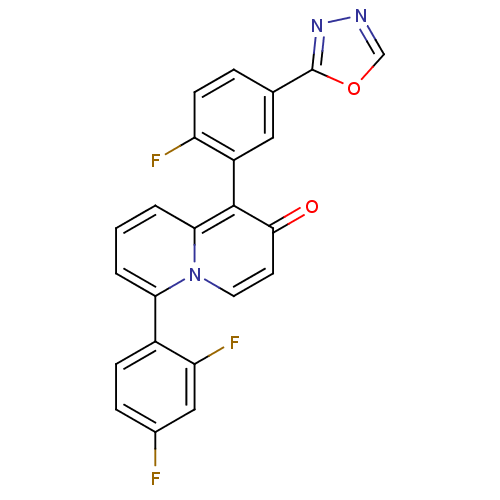


 3D Structure (crystal)
3D Structure (crystal)