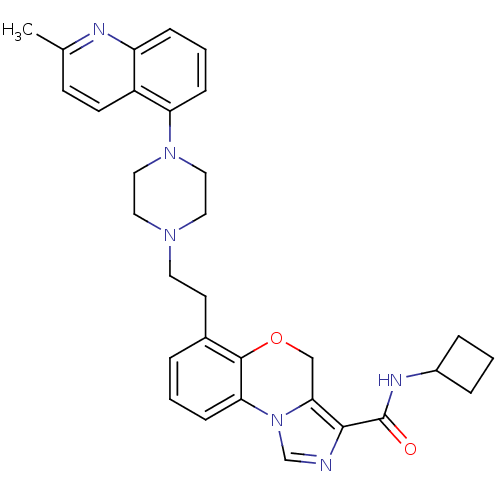
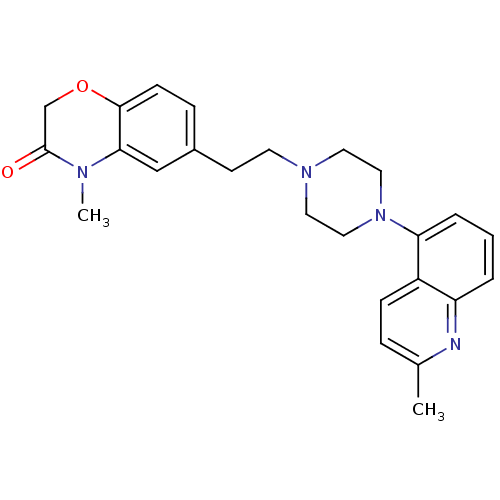
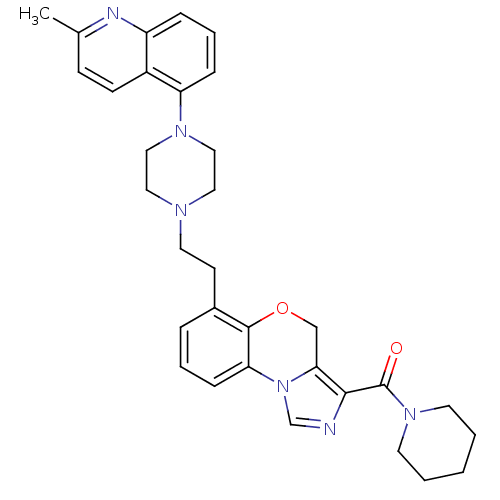
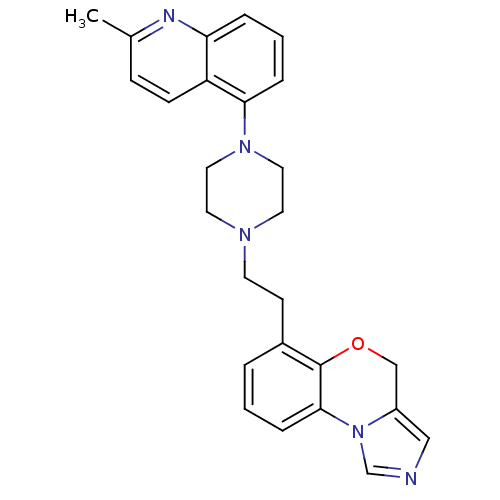
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 25.1nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 63.1nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 398nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 398nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 501nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 501nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 501nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 631nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 794nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 1.26E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 1.58E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 2.51E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 2.51E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 2.51E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 2.51E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 3.98E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 3.98E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 6.31E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: >7.94E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 7.94E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 1.26E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 3.16E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataKi: 3.16E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human recombinant CYP1A2 preincubated for 10 minsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human ERG tail current by patch clamp electrophysiology assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 1.58E+3nMAssay Description:Inhibition of human ERG tail current by patch clamp electrophysiology assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human recombinant CYP2C9 preincubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human recombinant CYP2C19 preincubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomes measured after incubationMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human recombinant CYP2C9 preincubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human recombinant CYP2C9 preincubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human recombinant CYP1A2 preincubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human recombinant CYP2C19 preincubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human recombinant CYP2C9 preincubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human recombinant CYP2C19 preincubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human recombinant CYP2C19 preincubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomes measured after incubationMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomes measured after incubationMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human recombinant CYP2C9 preincubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human recombinant CYP2C19 preincubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human recombinant CYPD6 preincubated for 10 minsMore data for this Ligand-Target Pair