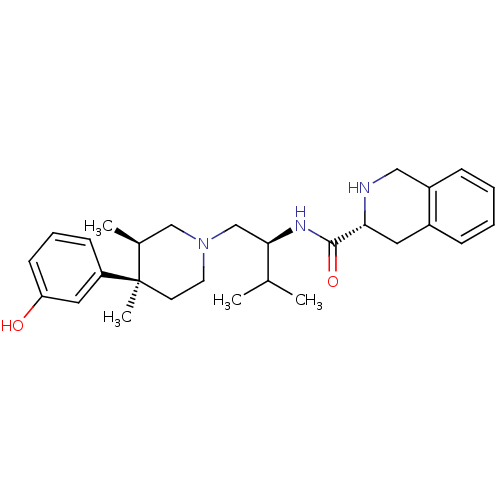
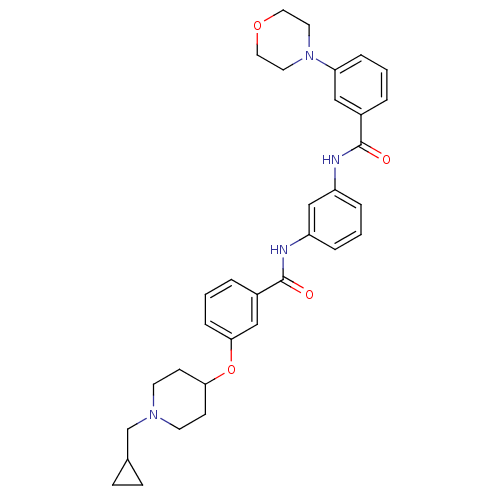
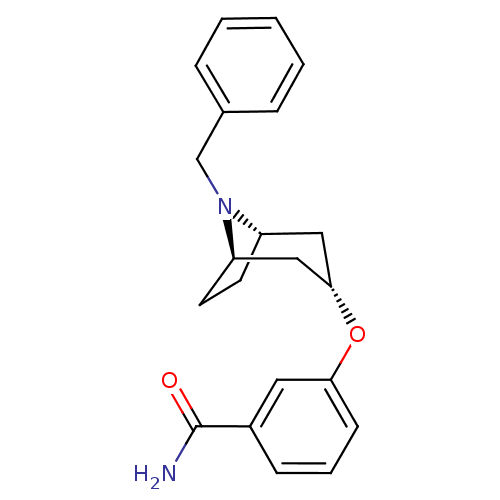
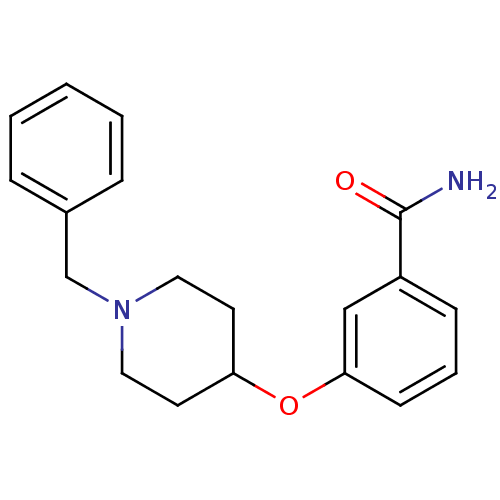
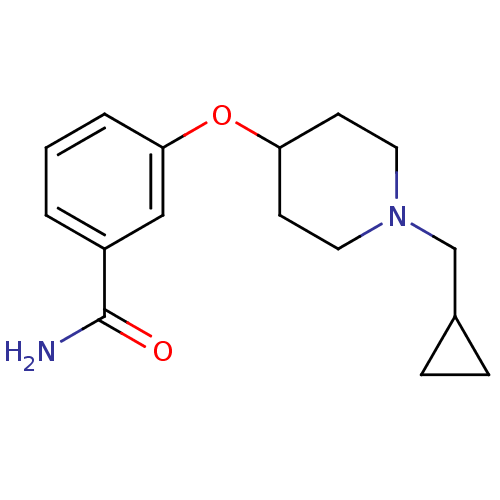
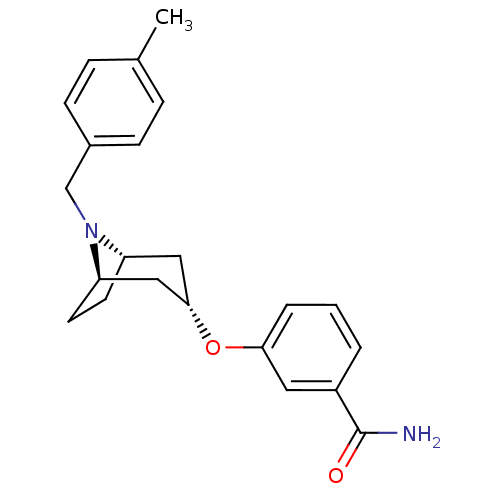
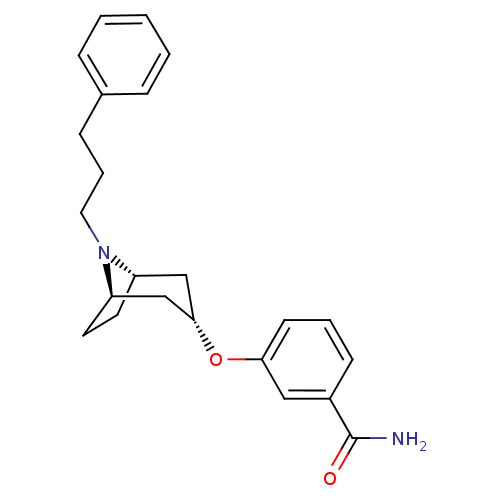
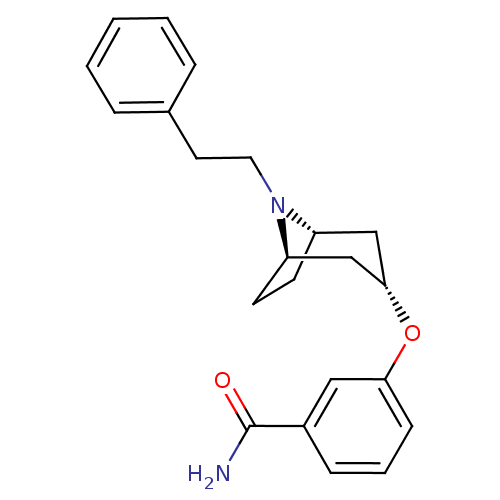
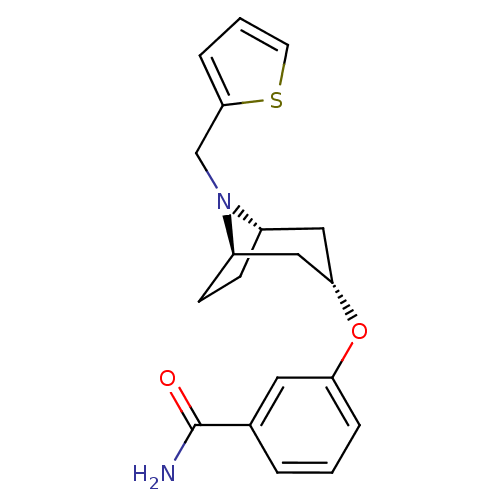
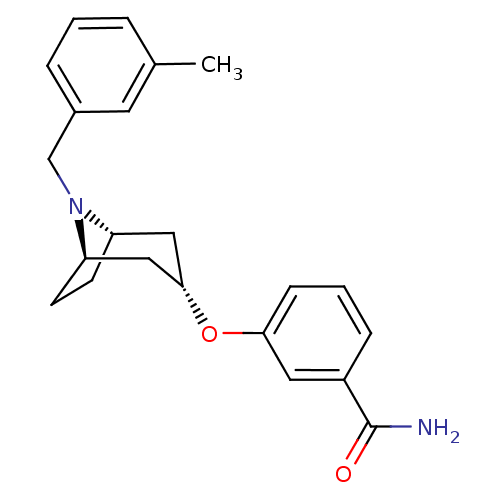
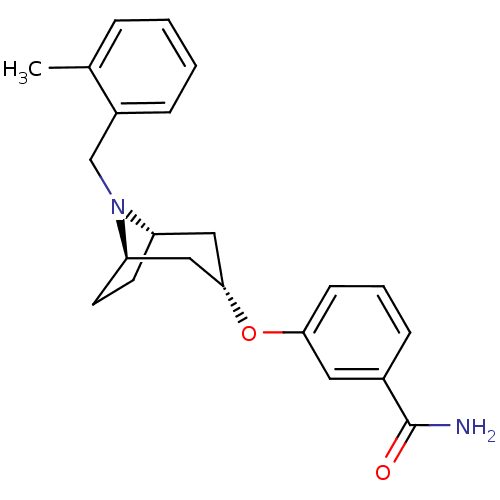
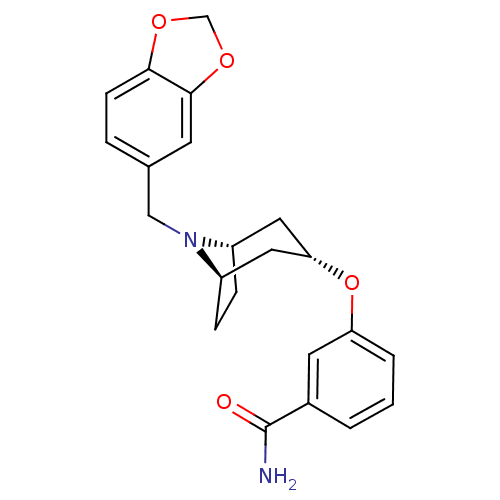
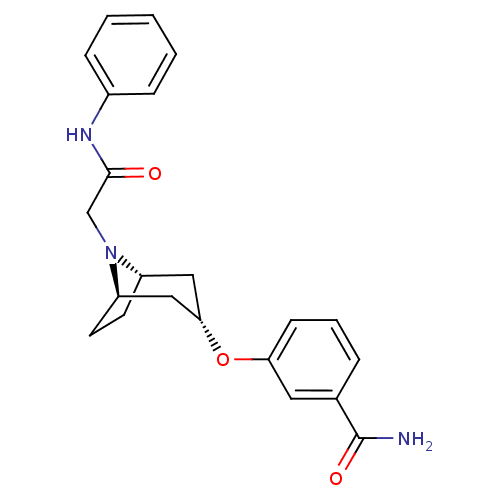
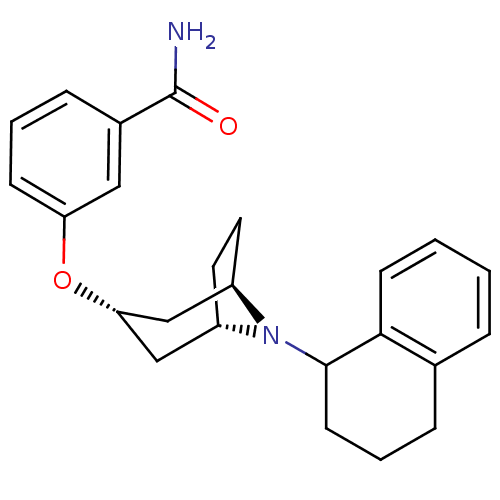
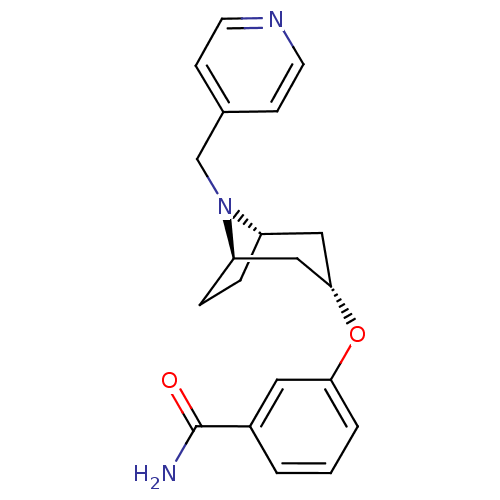
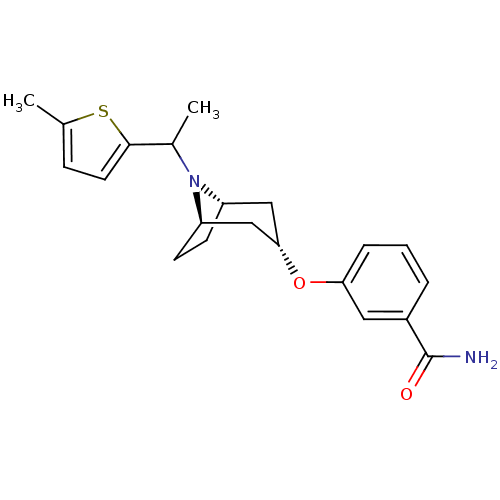
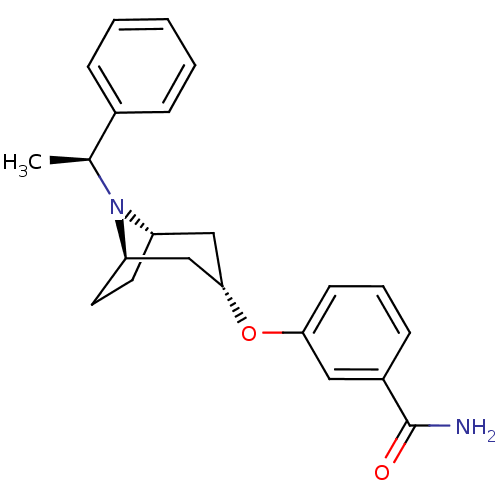
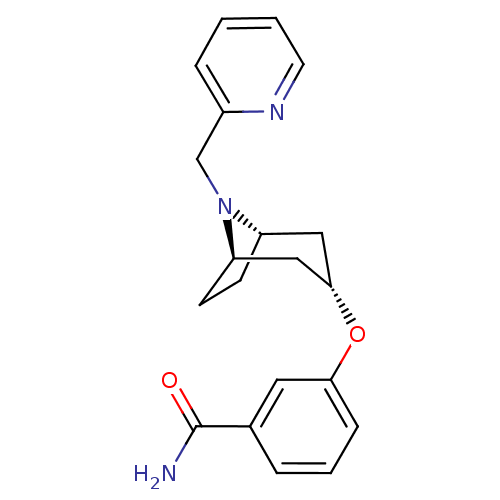
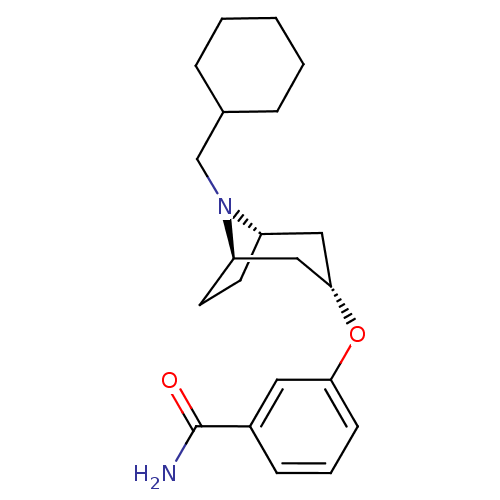
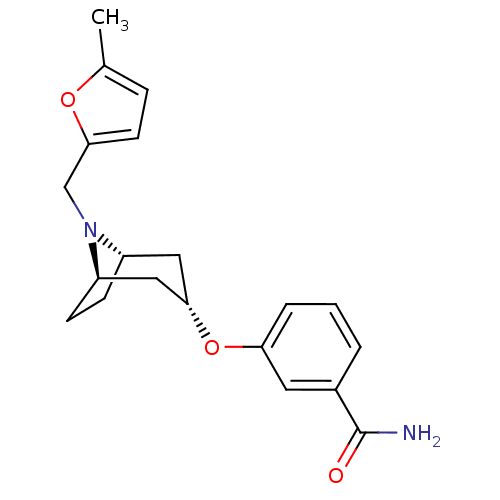
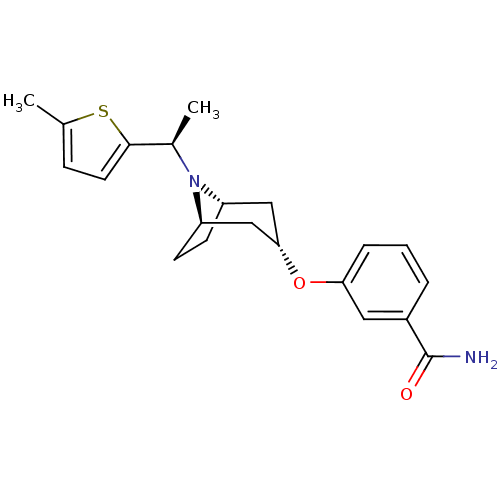
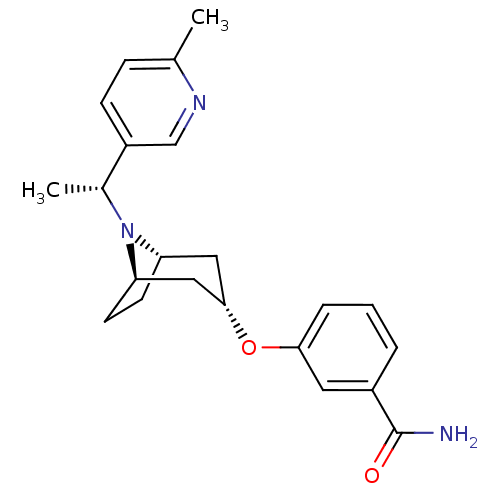
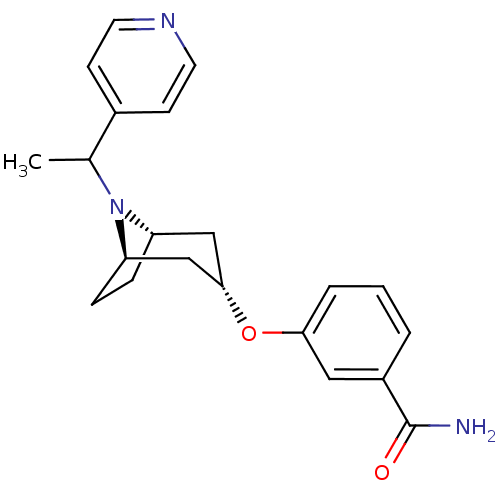
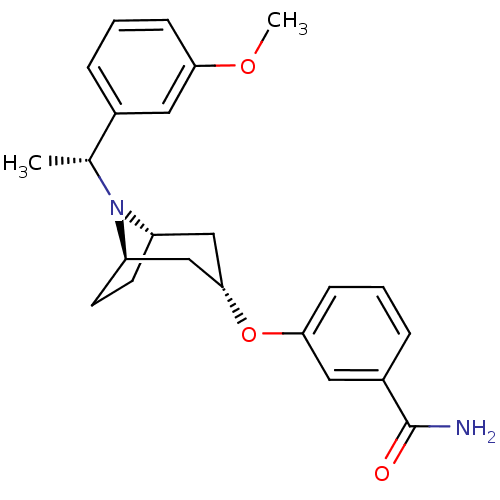
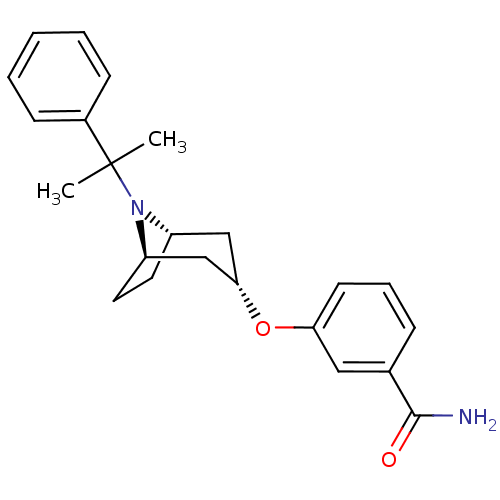
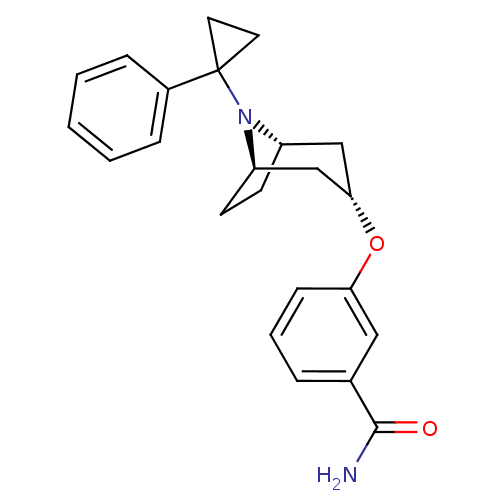
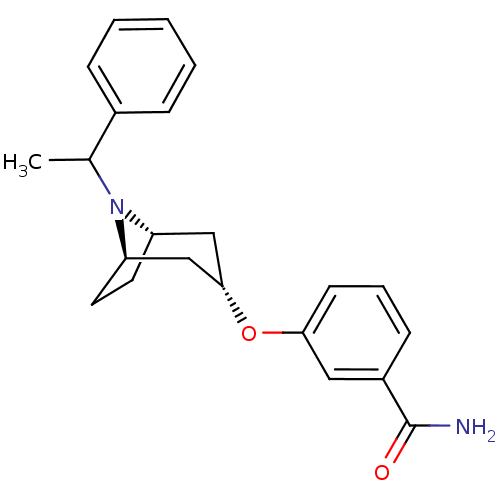
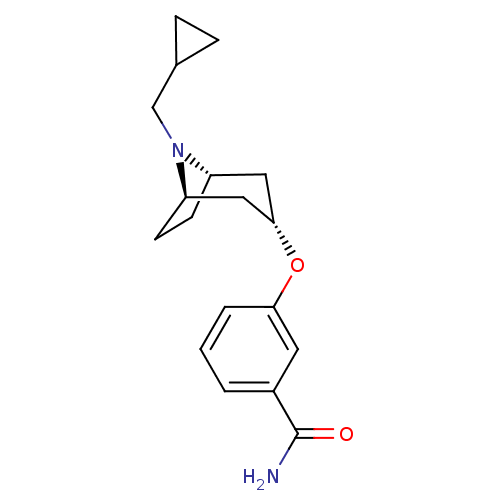
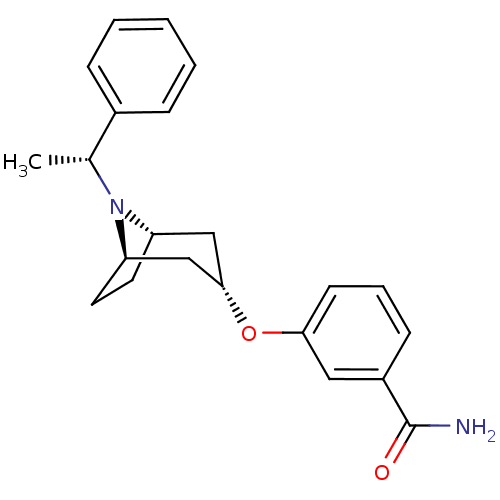
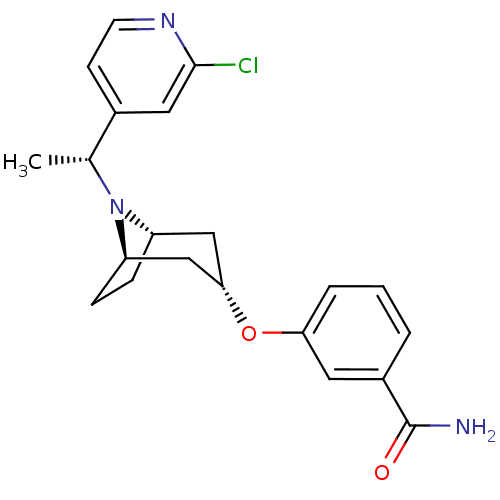
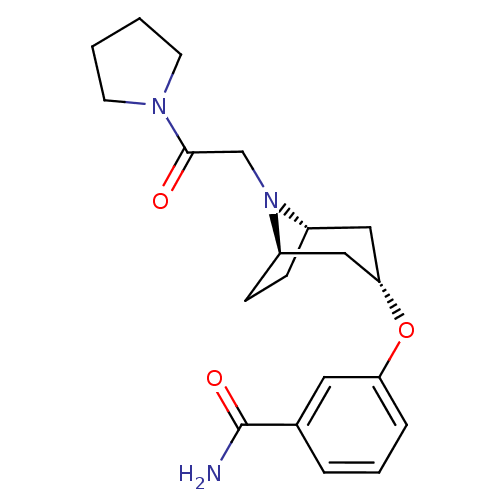
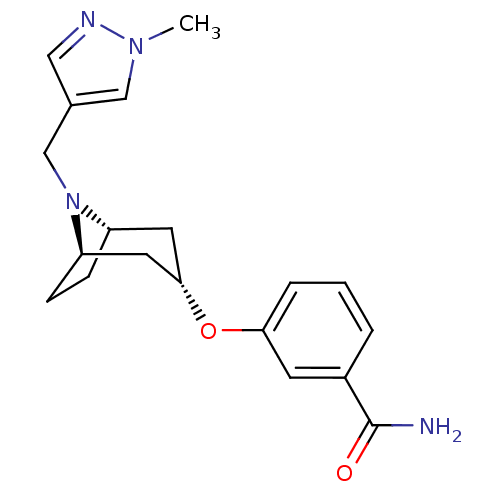
Affinity DataKi: 0.300nMAssay Description:Binding affinity to kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity to kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:Binding affinity to kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Binding affinity to kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 75nMAssay Description:Binding affinity to kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 146nMAssay Description:Binding affinity to kappa opioid receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.140nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.25nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.260nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.380nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.430nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.520nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.610nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.620nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.620nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.720nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.950nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.70nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.70nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.80nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6.60nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 7.30nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Antagonist activity at human delta opioid receptor assessed as inhibition of SNC80-stimulated [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca Pharmaceuticals
Curated by ChEMBL
AstraZeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Antagonist activity at human mu opioid receptor assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 49nMAssay Description:Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair