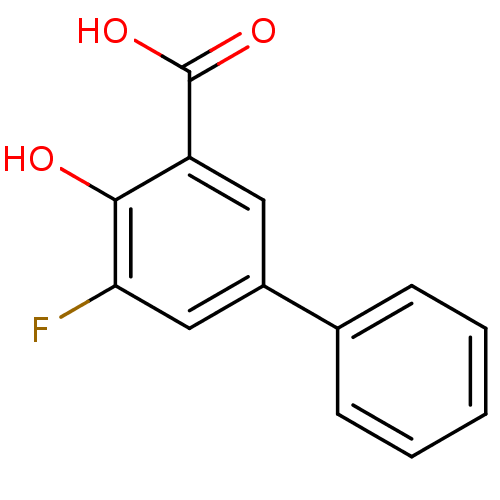
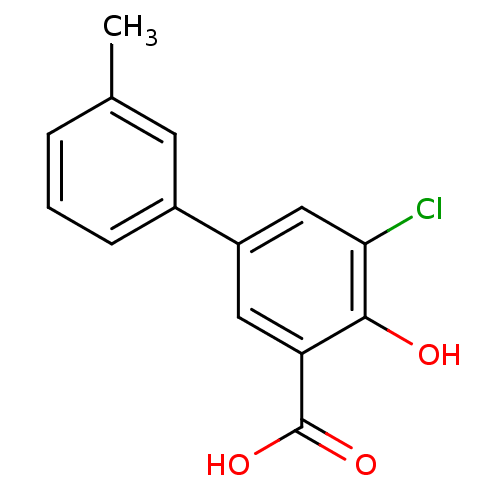
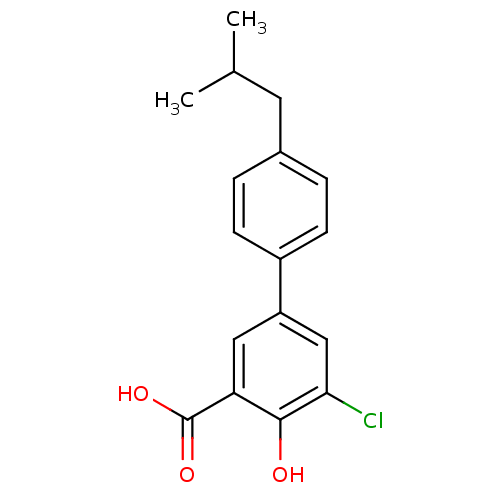
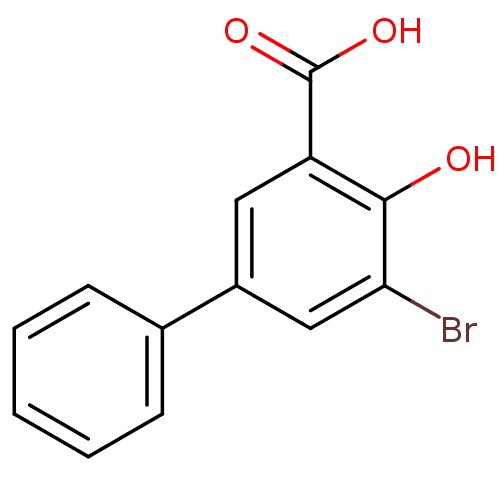
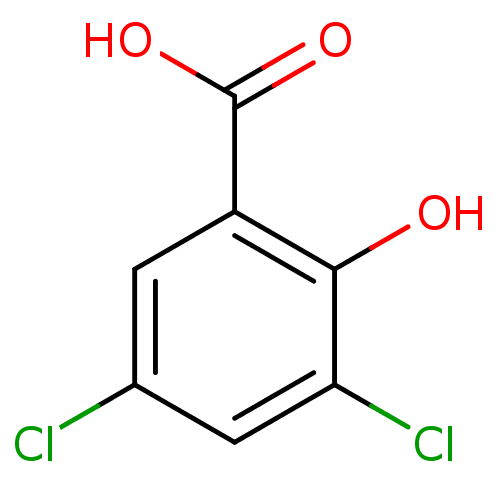
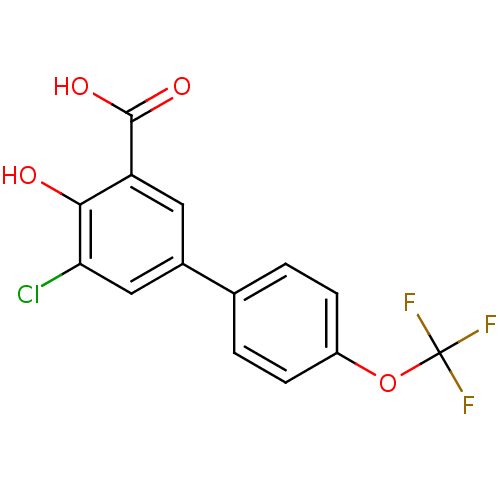
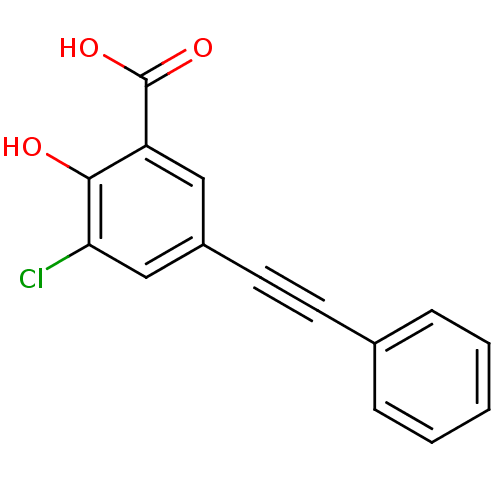
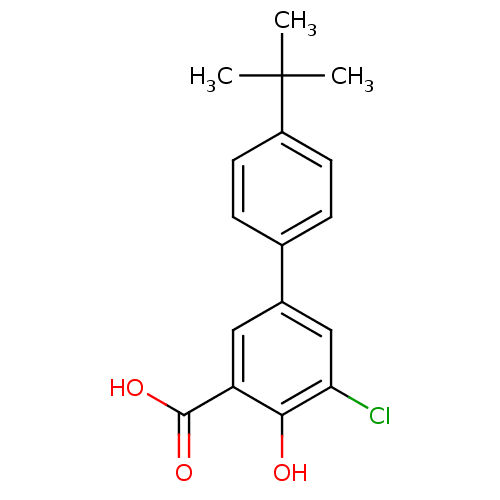
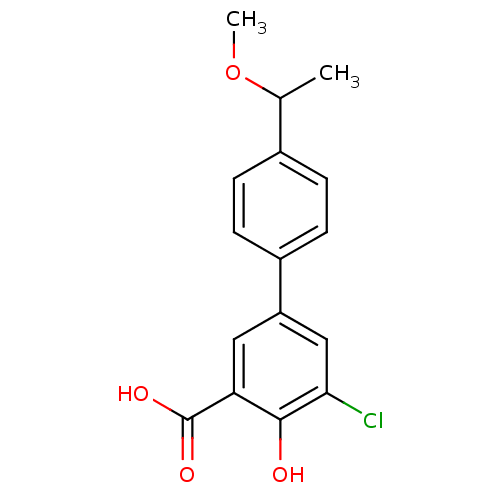
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 0.860nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 2.10nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 4.10nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 5.90nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 21nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 26nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 63nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 64nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 70nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 87nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 96nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 168nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 340nMAssay Description:Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 470nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human AKR1C1-mediated progesterone metabolism expressed in bovine aortic endothelial cells assessed as formation of 20alpha-hydroxyprog...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of human AKR1C1-mediated progesterone metabolism expressed in bovine aortic endothelial cells assessed as formation of 20alpha-hydroxyprog...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of human AKR1C1-mediated progesterone metabolism expressed in bovine aortic endothelial cells assessed as formation of 20alpha-hydroxyprog...More data for this Ligand-Target Pair

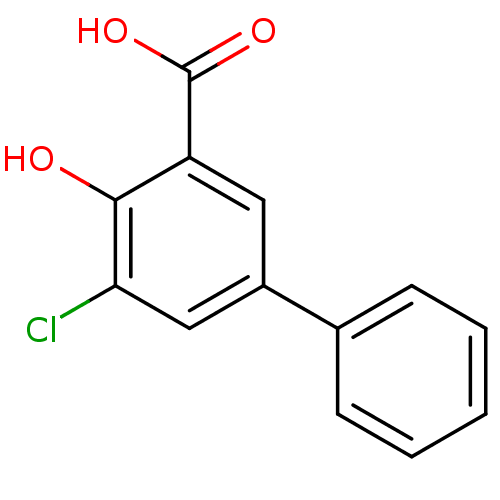
 3D Structure (crystal)
3D Structure (crystal)