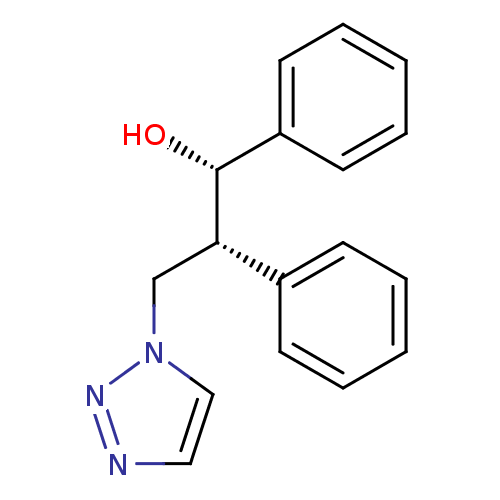
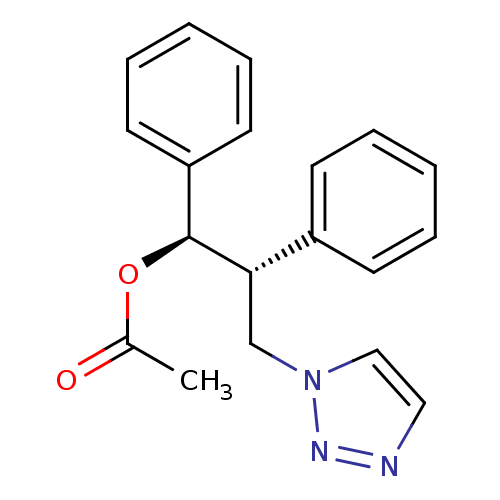
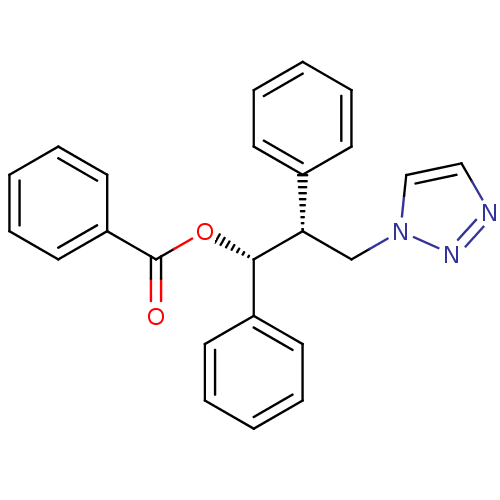
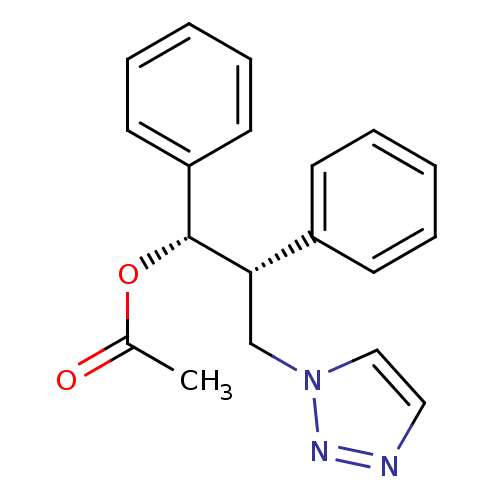
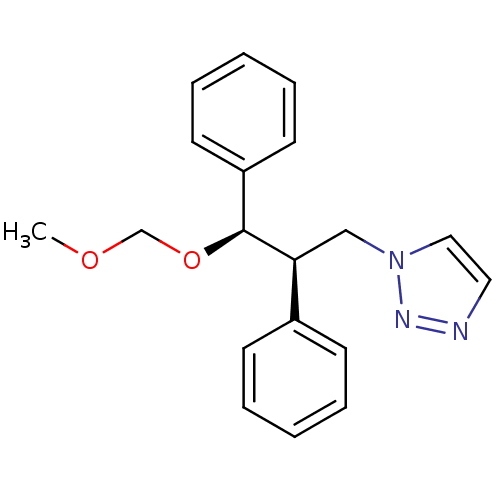
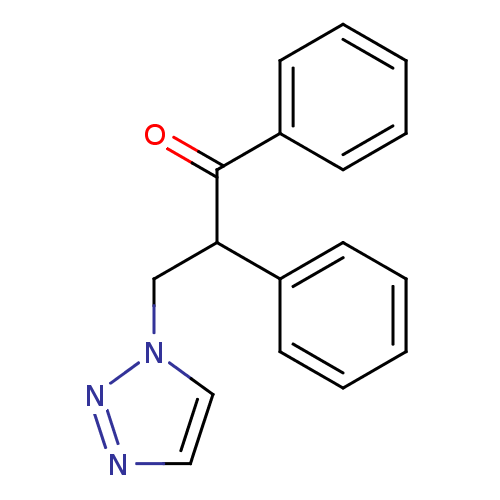
Affinity DataKi: 50nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 398nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 790nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 5.37E+3nMAssay Description:Inhibition of CYP1A1More data for this Ligand-Target Pair
Affinity DataKi: 3.09E+4nMAssay Description:Inhibition of CYP1A1More data for this Ligand-Target Pair
Affinity DataKi: 7.08E+5nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataKi: 1.12E+6nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair