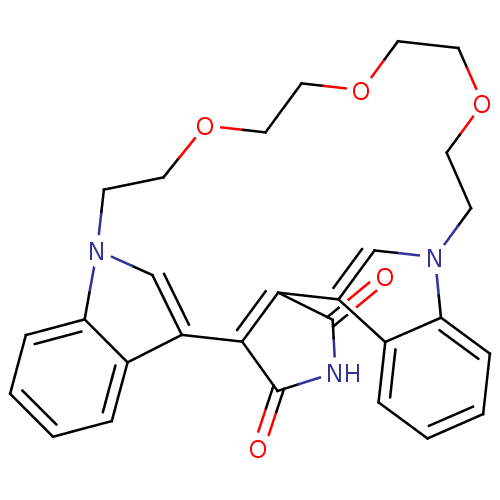
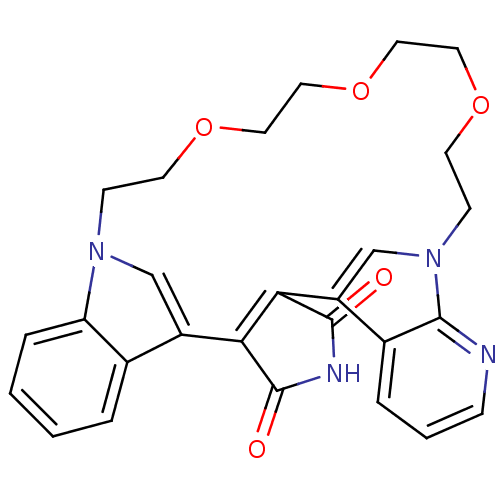
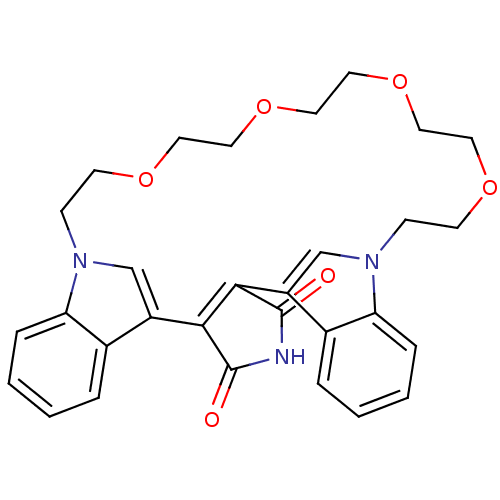
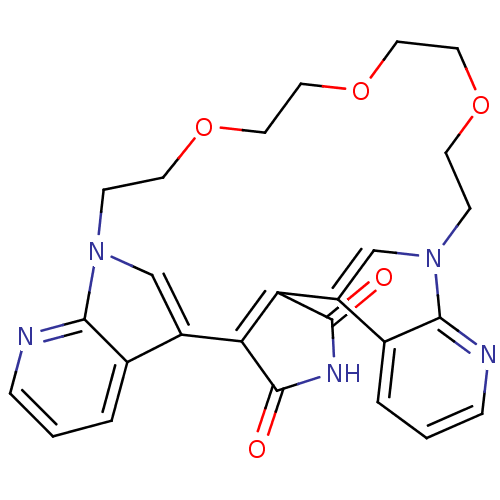
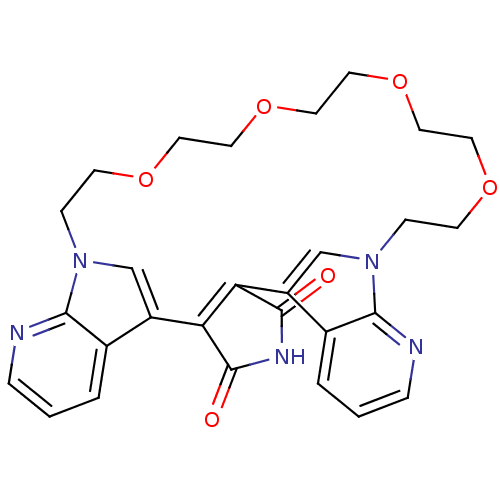
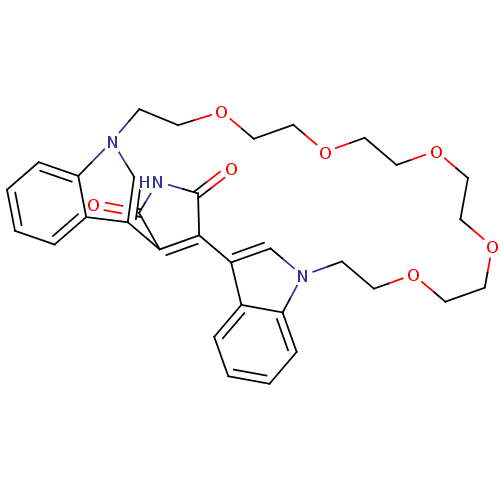
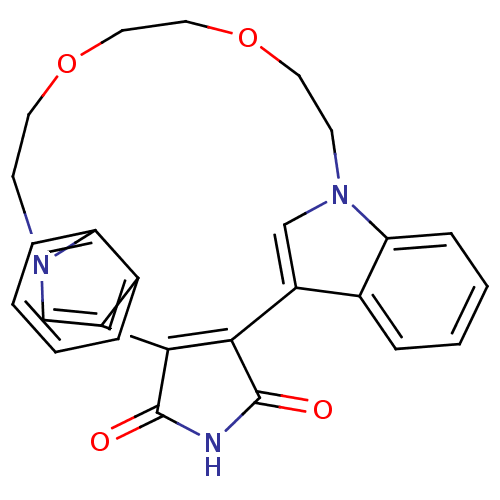
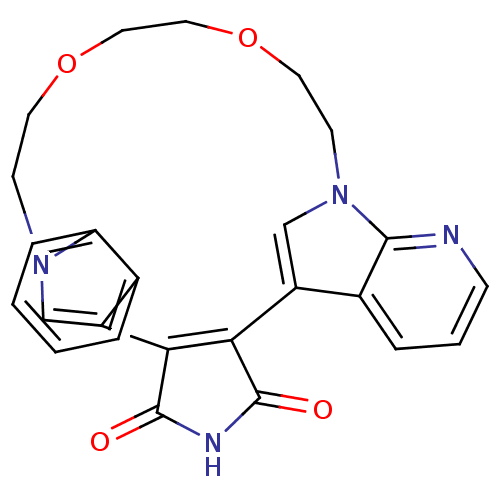
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of Protein kinase C beta 2More data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of Protein kinase C beta 2More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of Glycogen synthase kinase-3beta (GSK3-beta)More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibition of Glycogen synthase kinase-3beta (GSK3-beta)More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of Glycogen synthase kinase-3beta (GSK3-beta)More data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:Inhibition of Protein kinase C beta 2More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Inhibition of Glycogen synthase kinase-3beta (GSK3-beta)More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 51nMAssay Description:Inhibition of Glycogen synthase kinase-3beta (GSK3-beta)More data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Inhibition of Protein kinase C thetaMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 86nMAssay Description:Inhibition of Protein kinase C alphaMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 136nMAssay Description:Inhibition of Glycogen synthase kinase-3beta (GSK3-beta)More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 138nMAssay Description:Inhibition of Glycogen synthase kinase-3beta (GSK3-beta)More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of Glycogen synthase kinase-3beta (GSK3-beta)More data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 360nMAssay Description:Inhibition of Protein kinase C alphaMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 403nMAssay Description:Inhibition of Glycogen synthase kinase-3beta (GSK3-beta)More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 620nMAssay Description:Inhibition of Glycogen synthase kinase-3beta (GSK3-beta)More data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 673nMAssay Description:Inhibition of Protein kinase C alphaMore data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 835nMAssay Description:Inhibition of Protein kinase C thetaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 992nMAssay Description:Inhibition of Cyclin-dependent kinase 2 (CDK2)More data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.16E+3nMAssay Description:Inhibition of Protein kinase C beta 2More data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.25E+3nMAssay Description:Inhibition of Cyclin-dependent kinase 1More data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.34E+3nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.44E+3nMAssay Description:Inhibition of Cyclin-dependent kinase 2 (CDK2)More data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.67E+3nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.73E+3nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.43E+3nMAssay Description:Inhibition of Cyclin-dependent kinase 1More data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 5.17E+3nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Calcium/calmodulin-dependent protein kinase IIMore data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Calcium/calmodulin-dependent protein kinase IIMore data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Calcium/calmodulin-dependent protein kinase IIMore data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Protein kinase C beta 2More data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Calcium/calmodulin-dependent protein kinase IIMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Protein kinase C alphaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Cyclin-dependent kinase 1More data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Cyclin-dependent kinase 2 (CDK2)More data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Protein kinase C thetaMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Protein kinase C alphaMore data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Cyclin-dependent kinase 1More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Cyclin-dependent kinase 2 (CDK2)More data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Protein kinase C thetaMore data for this Ligand-Target Pair