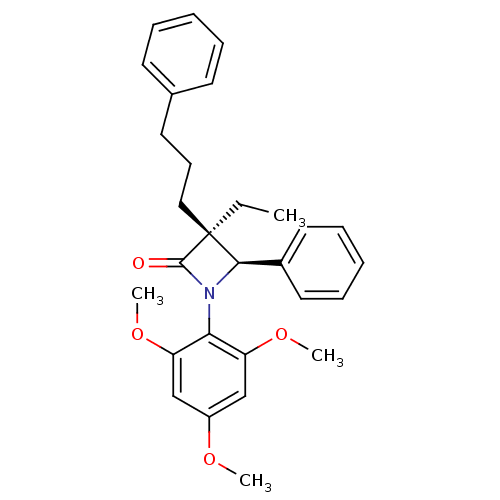
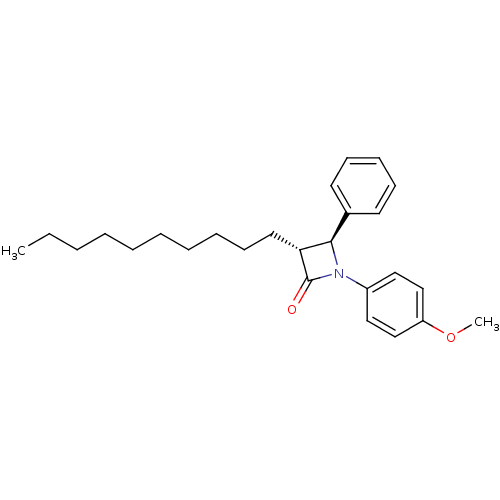
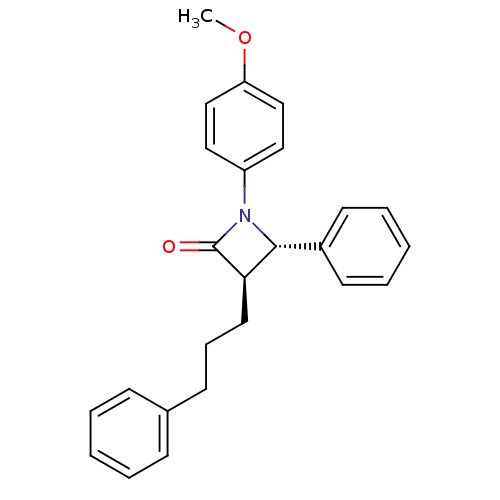
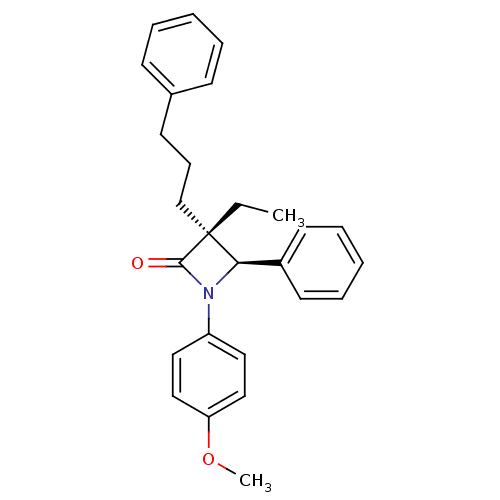
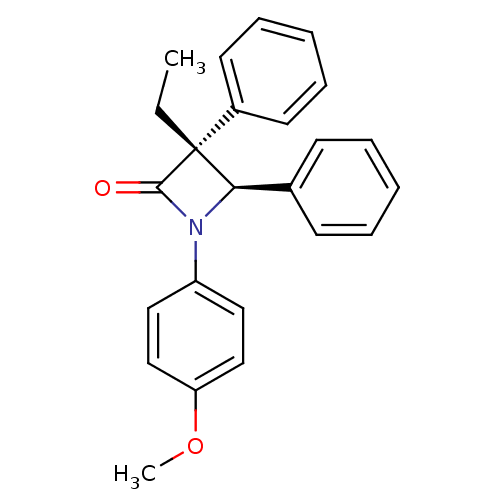
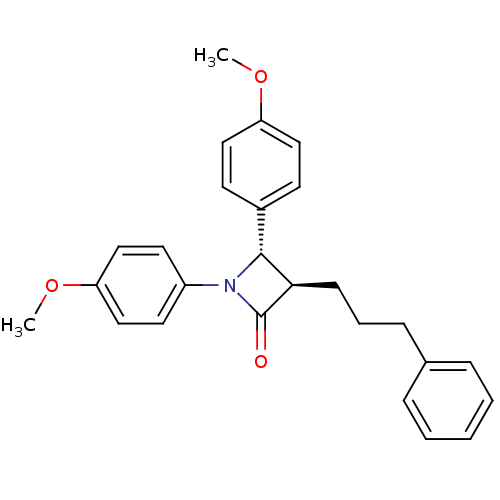
Affinity DataKi: 0.700nMAssay Description:Binding affinity to Sigma opioid receptor type 1 in guinea pig brain homogenate with 0.5 nM of [3H](+)-PENT as radioligandMore data for this Ligand-Target Pair
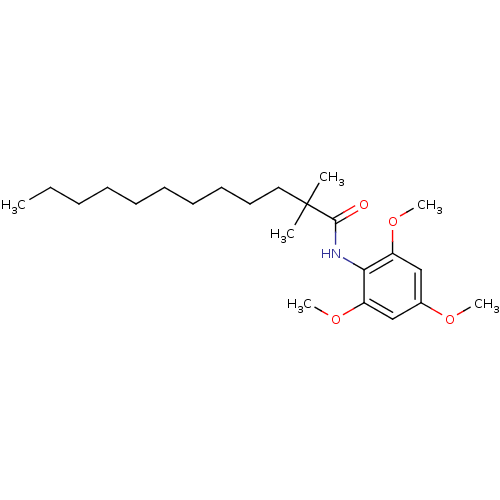
Affinity DataKi: 4.5nMAssay Description:Binding affinity to Sigma opioid receptor type 2 in guinea pig brain homogenate with 4 nM of [3H](+)-DTG as radioligandMore data for this Ligand-Target Pair
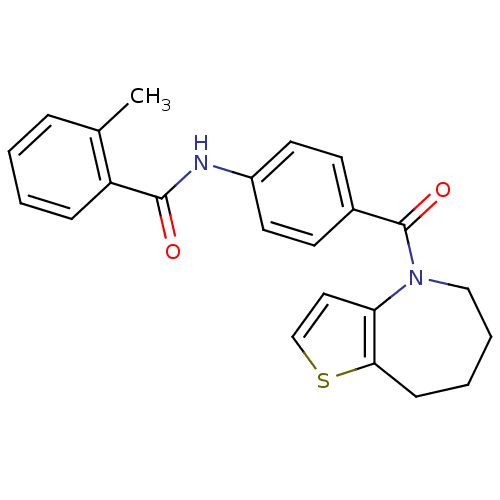
Affinity DataKi: 220nMAssay Description:Compound was evaluated for its inhibitory constant against 3-dehydroquinate synthaseMore data for this Ligand-Target Pair
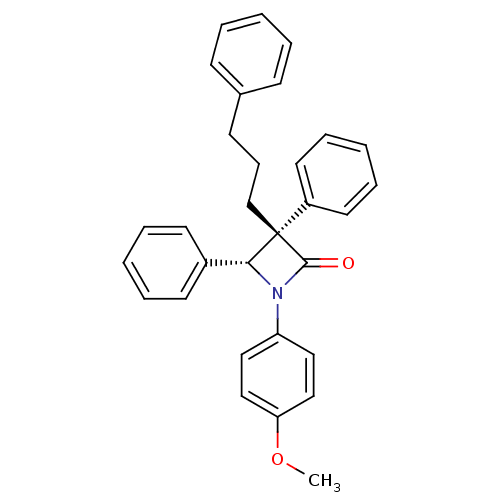
Affinity DataKi: 250nMAssay Description:Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12)More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.70nMAssay Description:In vitro binding affinity against angiotensin II AT-2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 66nMAssay Description:In vitro binding affinity against angiotensin II AT-2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 310nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Binding affinity towards recombinant FK506 binding protein 12 to determine the FKBP binding property of the compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+3nMAssay Description:Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medullaMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+3nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+4nMAssay Description:Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT)More data for this Ligand-Target Pair


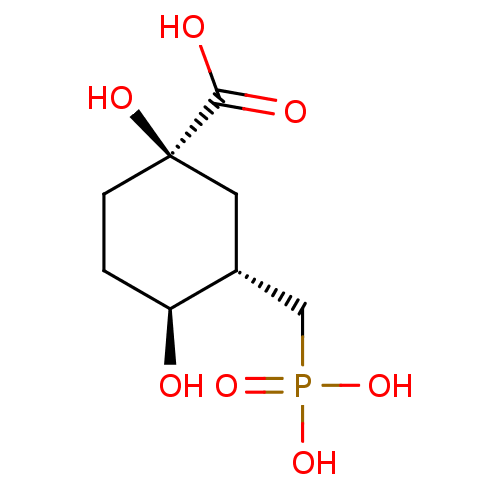

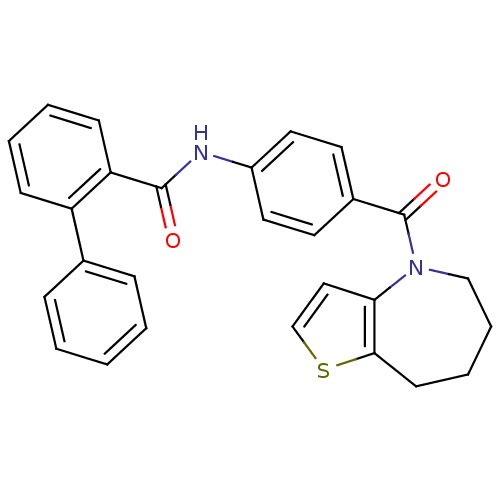
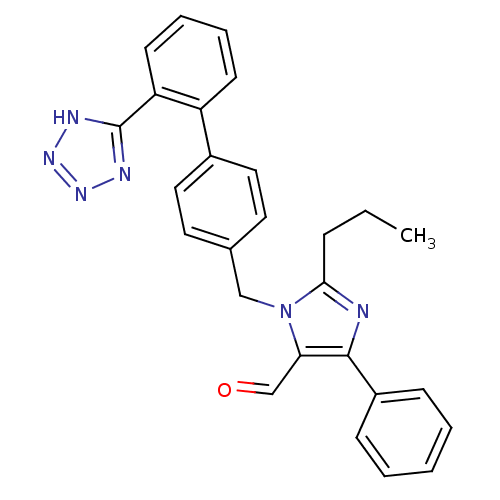
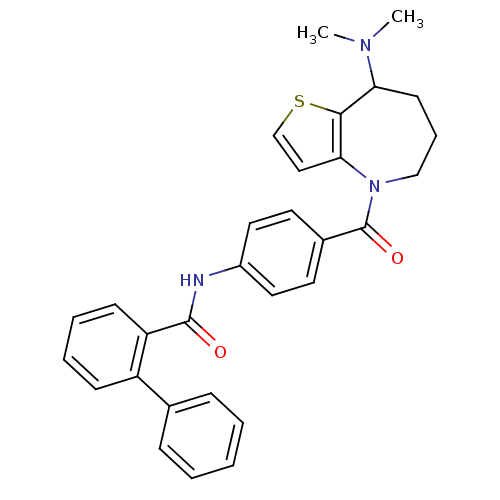
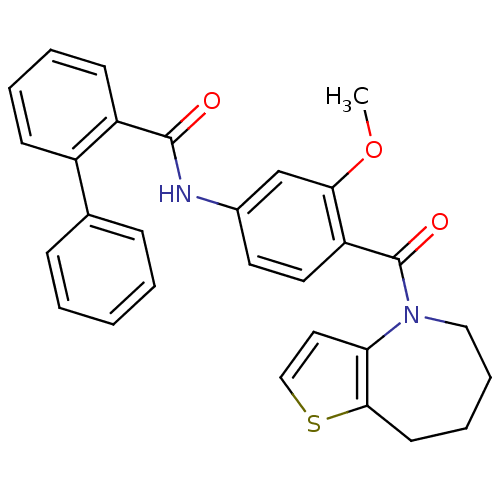
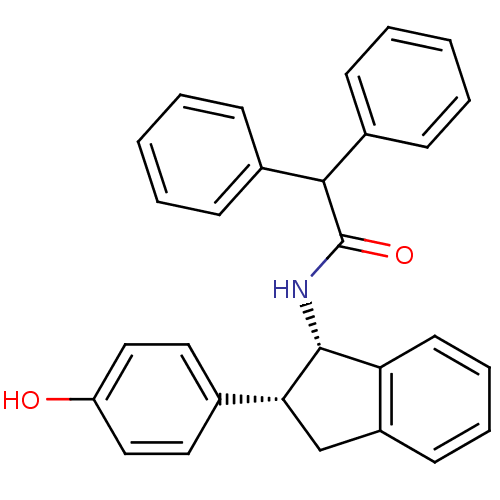
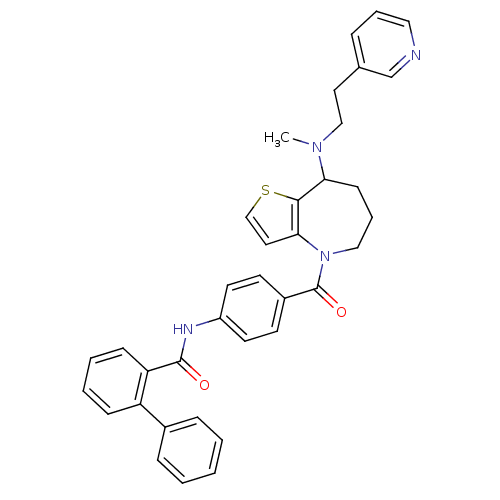
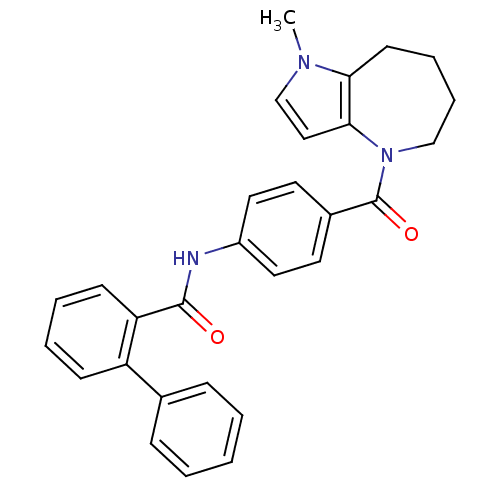
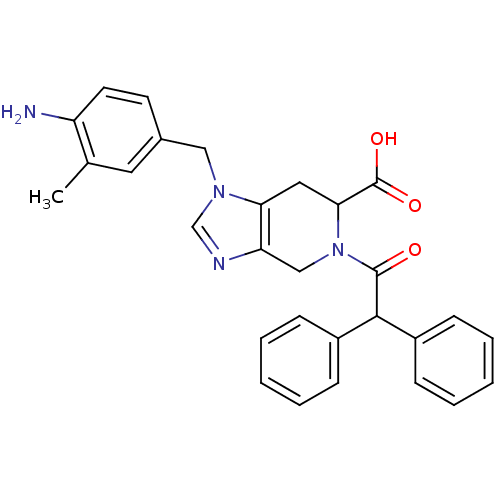

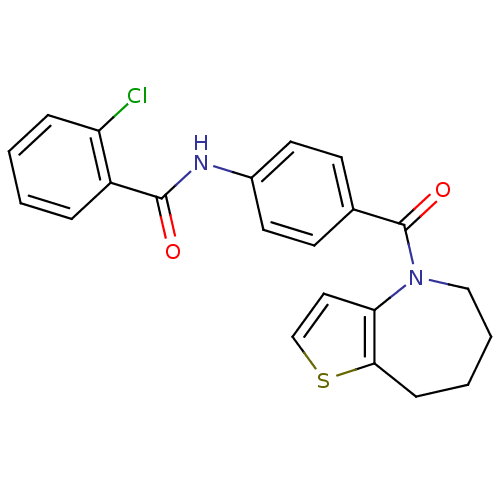
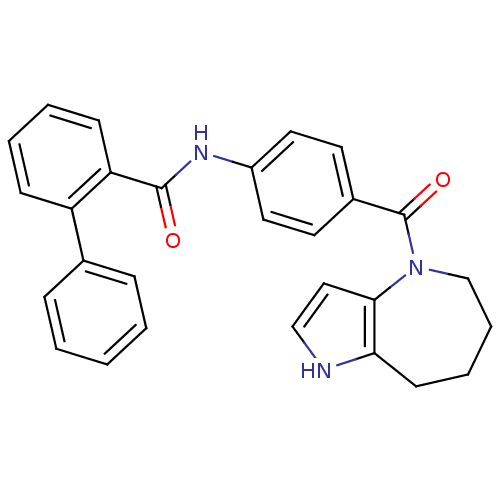
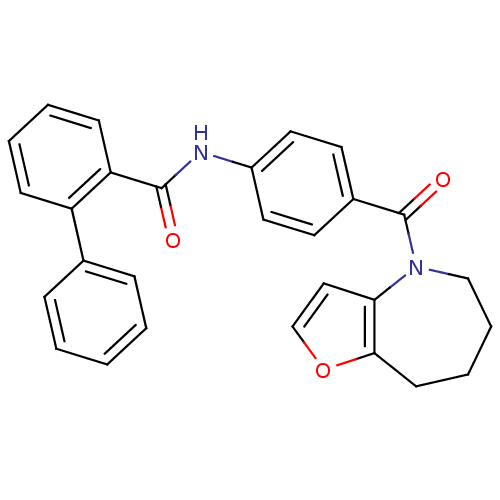

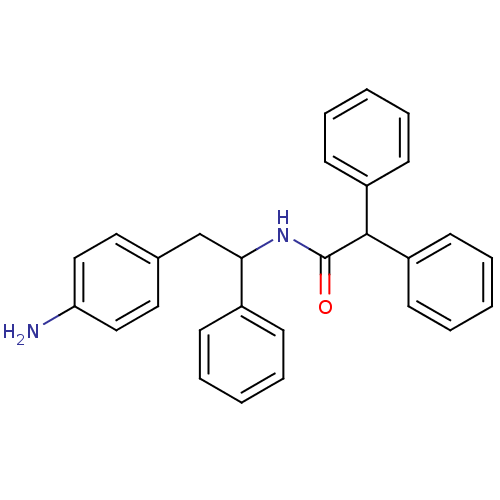
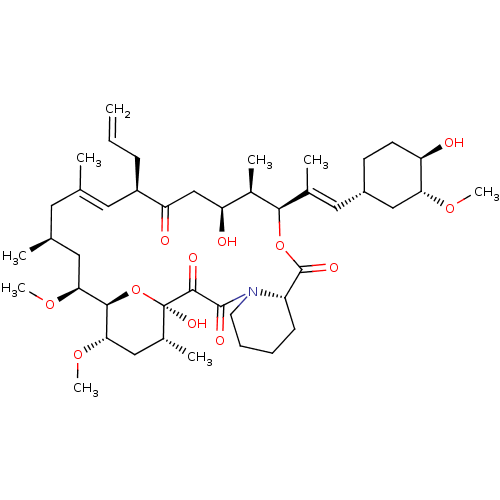
 3D Structure (crystal)
3D Structure (crystal)