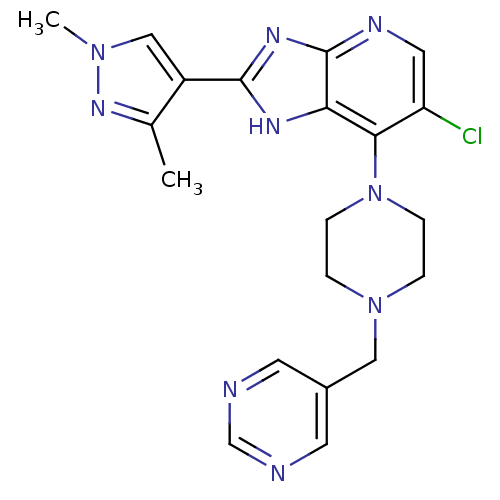
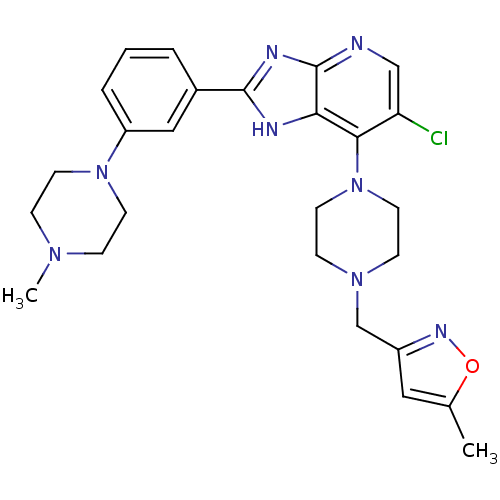
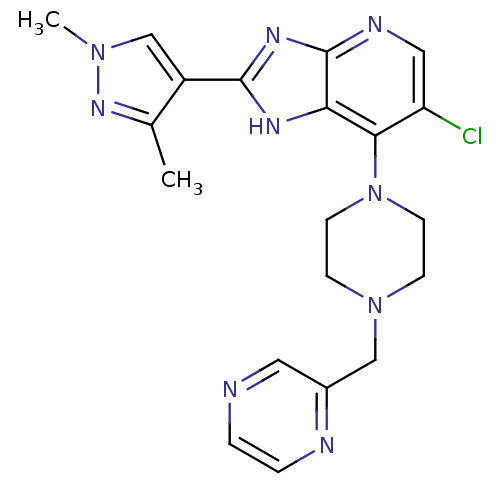
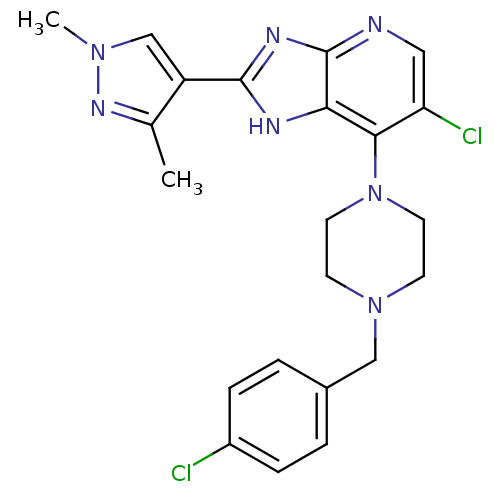
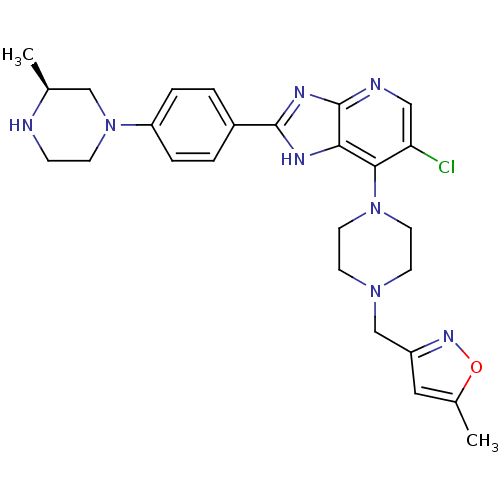
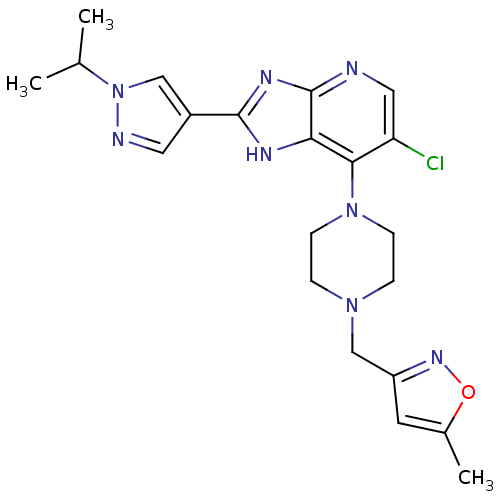
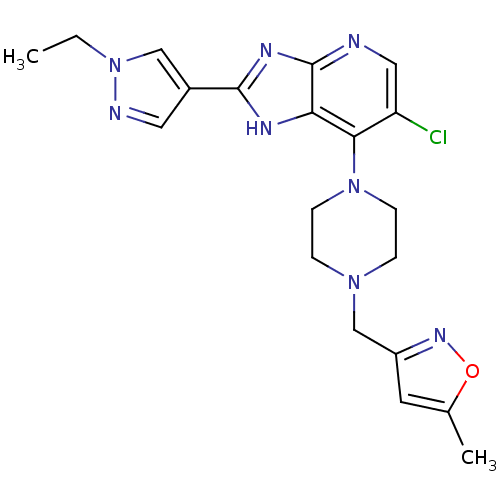
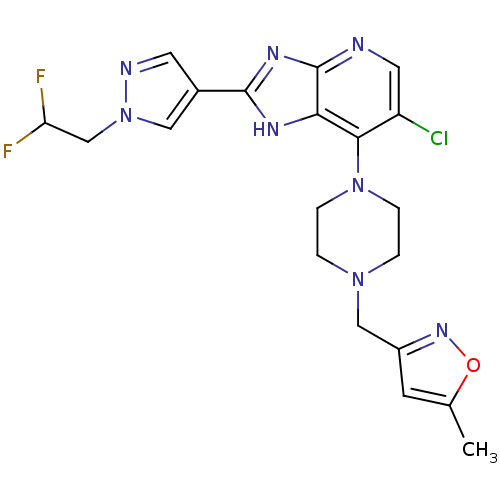
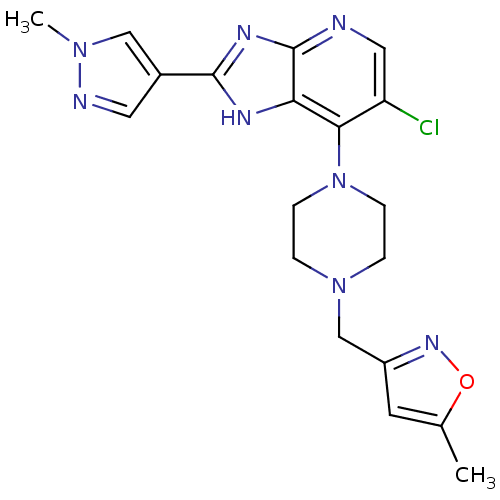
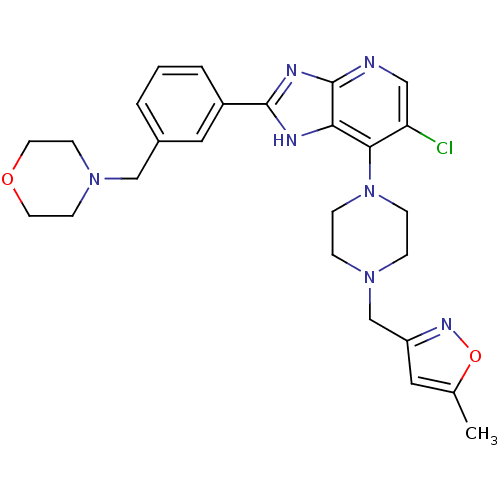
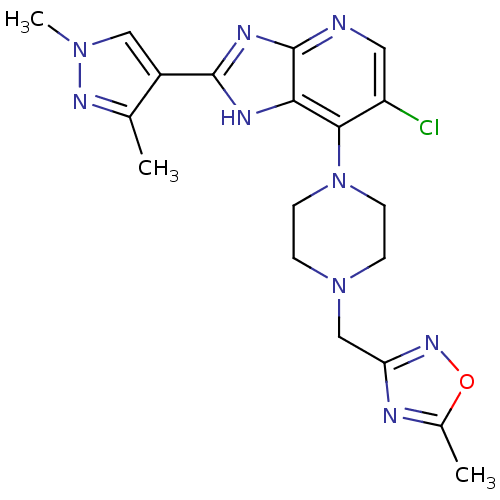
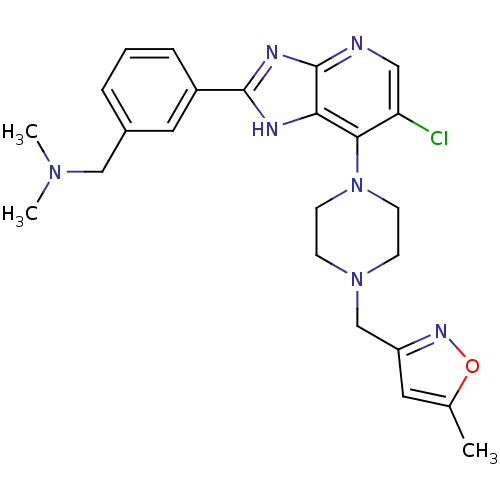
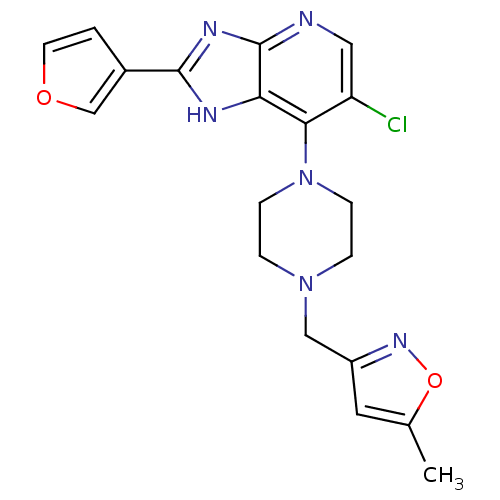
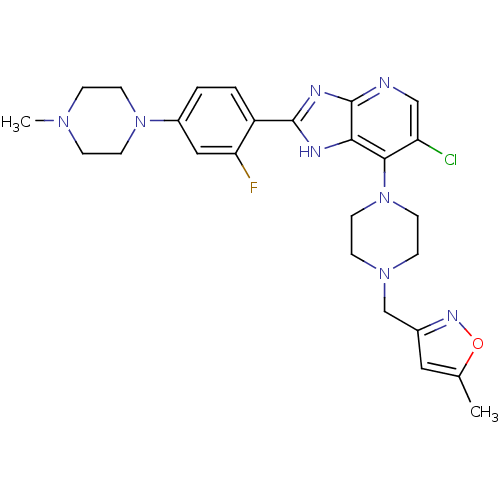
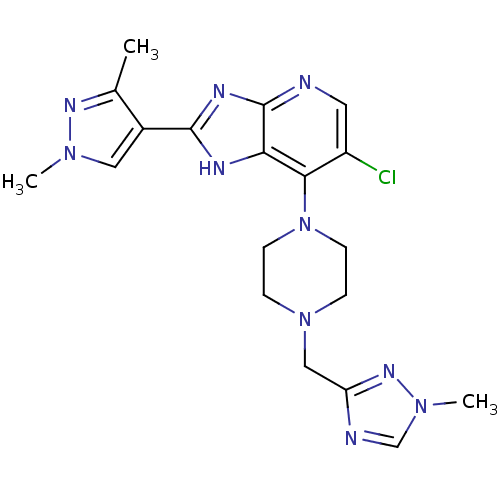
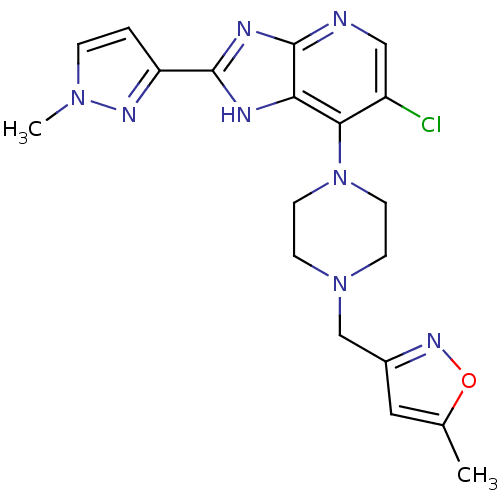
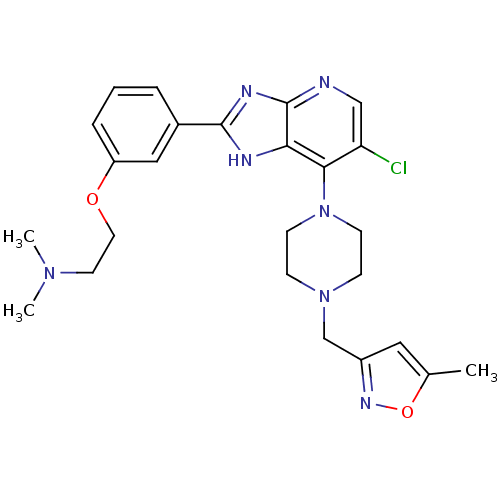
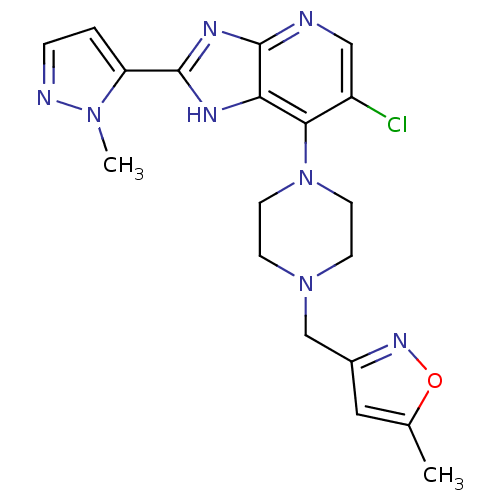
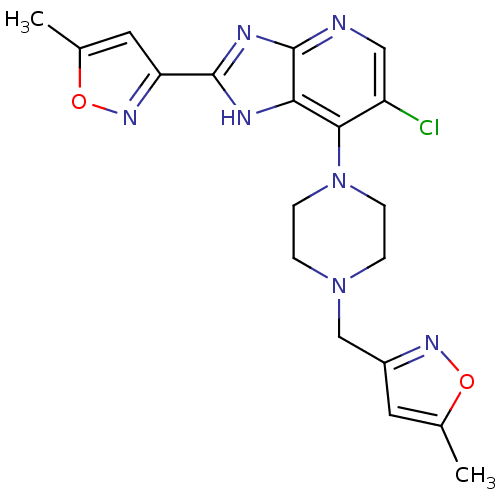
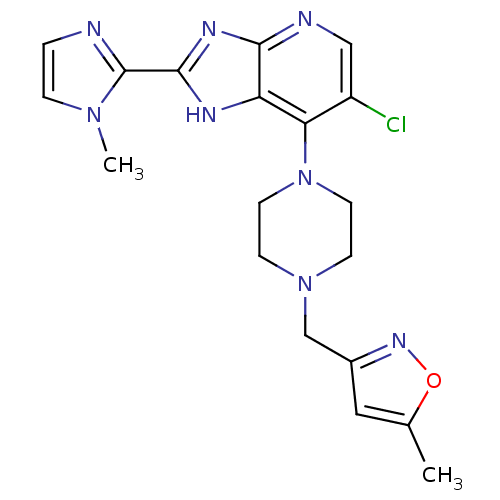
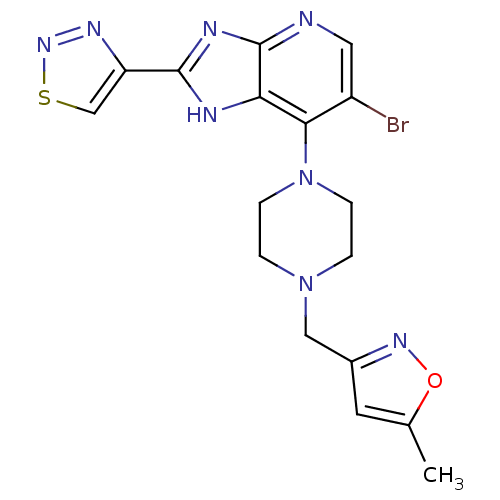
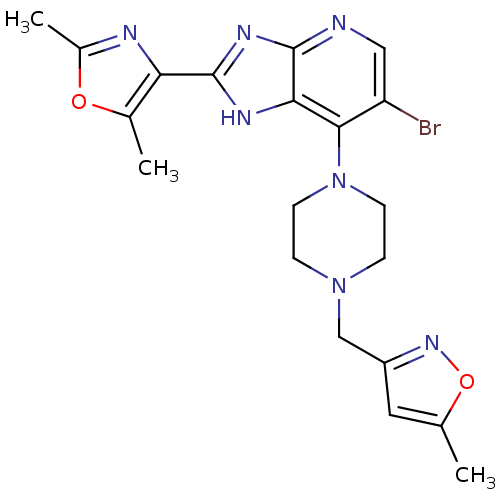
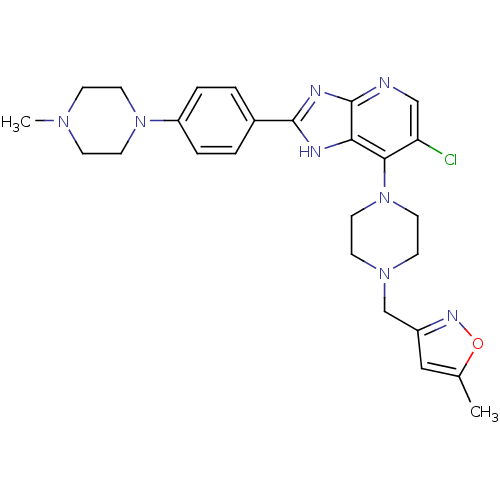
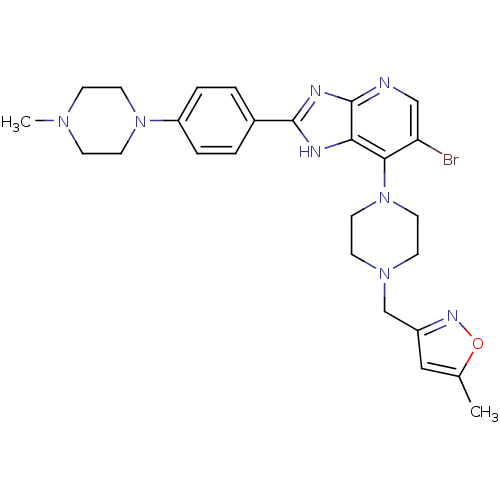
Affinity DataIC50: 4nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of Aurora A kinase autophosphorylation at T288 in human HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 52nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 87nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 93nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 118nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 147nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 148nMAssay Description:Inhibition of Aurora B kinase-mediated Histone H3 phosphorylation at S10 in human HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 434nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 564nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Binding affinity to Aurora A kinaseMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of human ERG by whole-cell voltage clampingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human ERG by whole-cell voltage clampingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human ERG by whole-cell voltage clampingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 6.30E+3nMAssay Description:Inhibition of human ERG by whole-cell voltage clampingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibition of human ERG by whole-cell voltage clampingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibition of human ERG by whole-cell voltage clampingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human liver microsome CYP1A2 by LC-MS-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human liver microsome CYP3A4 by LC-MS-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human liver microsome CYP2D6 by LC-MS-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human liver microsome CYP2C9 by LC-MS-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human liver microsome CYP2C19 using mephenytoin as a substrate by LC-MS-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human liver microsome CYP2A6 by LC-MS-MS analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of human ERG by whole-cell voltage clampingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human ERG by whole-cell voltage clampingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of human ERG by whole-cell voltage clampingMore data for this Ligand-Target Pair
Affinity DataKd: 48nMAssay Description:Binding affinity to Aurora B kinaseMore data for this Ligand-Target Pair

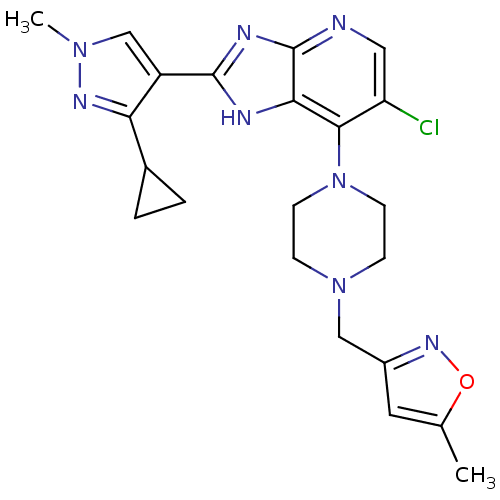
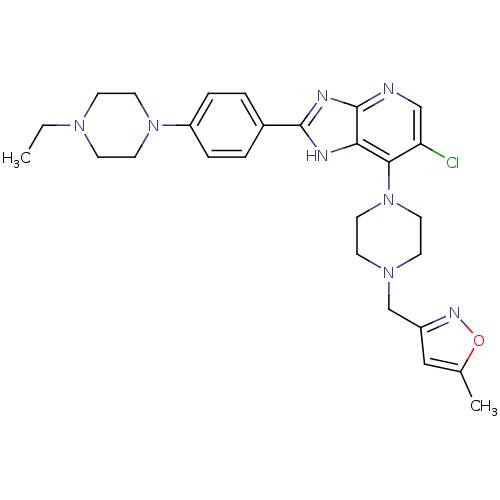
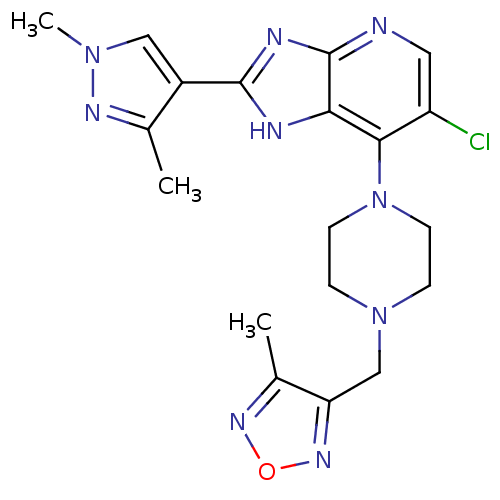
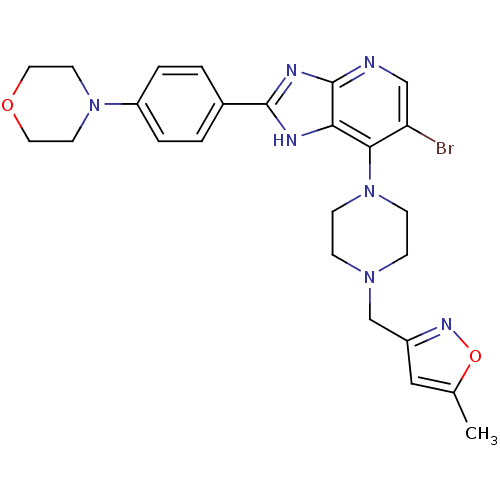
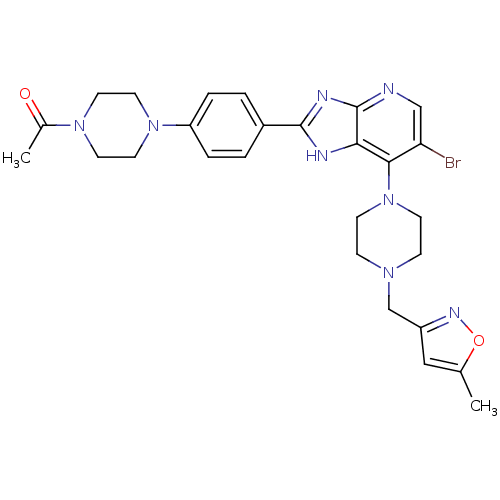
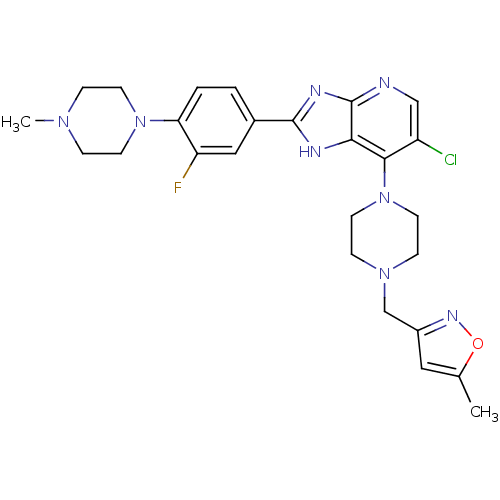
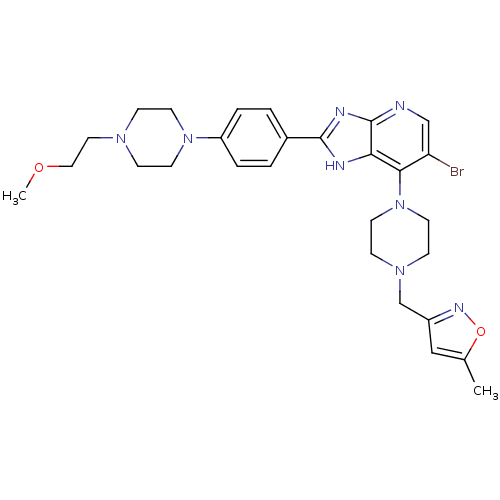
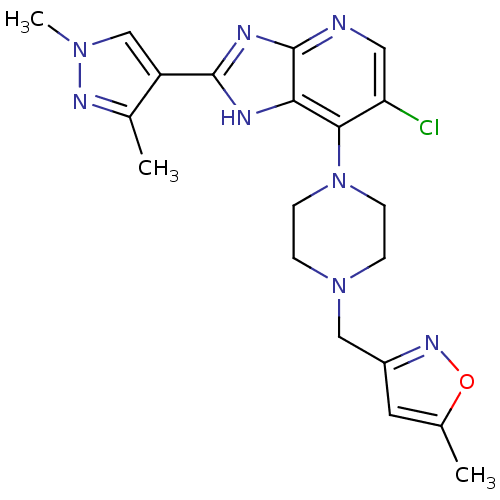
 3D Structure (crystal)
3D Structure (crystal)